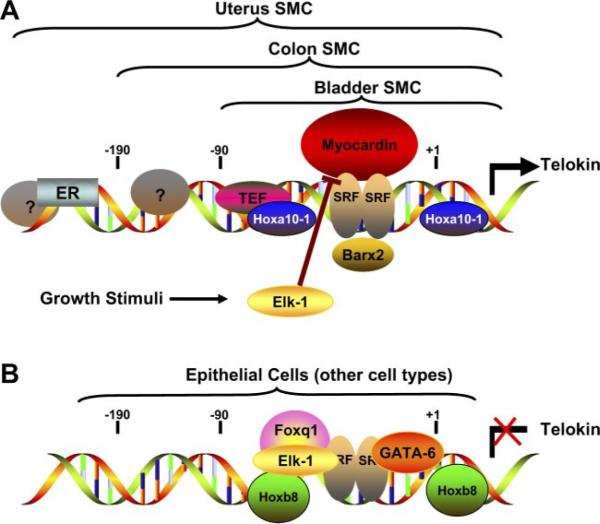
Fig. 4.
Telokin promoter regulation in smooth muscle and nonmuscle cells. A schematic model is shown of the transcription factors that bind to the telokin promoter in smooth muscle cells (SMC) (A) and in epithelial or nonmuscle cells (B). Analysis of promoter fragments in transgenic mice has suggested that the core of the telokin promoter, which includes a CArG box and adjacent AT-rich region, is required for expression in all smooth muscle tissues (38), and a −90 to +180-bp fragment that includes this region is sufficient for expression in bladder smooth muscle cells (33). Additional unidentified elements between −90 and −190 bp are required for telokin expression in smooth muscle cells in the gastrointestinal tract (33). In most higher mammals, with the exception of rodents, the estrogen receptor (ER) and perhaps other proteins that bind to more distal sequences are required for expression in uterine smooth muscle cells (71). Transcription factors that have been found to bind to the core of the telokin promoter directly [Hoxa10-1, thyrotroph embryonic factor (TEF), and serum response factor (SRF) (16, 30, 97)] or indirectly through their association with SRF [Barx2 and Myocardin (28, 96)] and activate the promoter are shown in A. Transcriptional inhibitors that repress the activity of the telokin promoter in epithelial cells or other nonmuscle cell types are shown in B. The relative contribution of each of the transcription factors to the transcriptional activity of the telokin promoter is likely to be different in smooth muscle cells from distinct tissues and in different nonmuscle cells. GATA-6 may inhibit telokin promoter activity in vascular smooth muscle cells by competing with myocardin for binding to SRF. Similarly, because Elk-1 also can compete with myocardin for binding to SRF, growth factor stimulation of smooth muscle cells may result in activation of Elk-1 and inhibition of telokin expression (98).