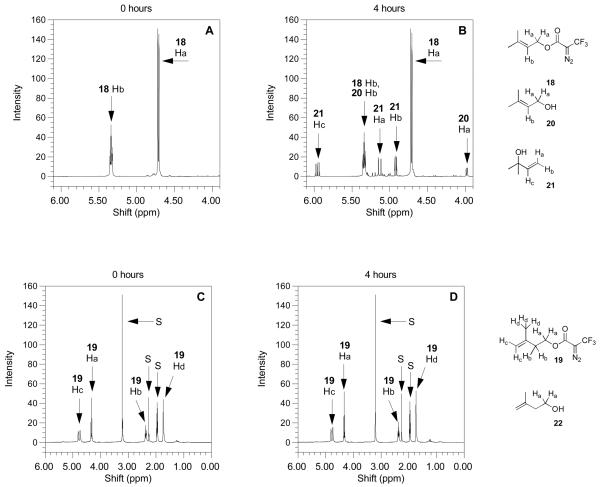
Figure 8.
1H-NMR spectra of DATFP-containing model esters obtained under mild acidolytic conditions. Spectra on the left are the compounds before heating and spectra on the right are the compounds after heating at 100°C for 4 h. (A) Compound 18 treated under acidic conditions (CD3CN/D20 10%/TFA-d 0.1%) before heating. (B) Compound 18 treated under acidic conditions (CD3CN/D20 10%/TFA-d 0.1%) after heating to 100°C for 4 h. The appearance of three new doublets of doublets after heating indicates the presence of 21. (C) Compound 19 treated under acidic conditions (CD3CN/D20 10%/TFA-d 0.1%) before heating. (D) Compound 18 treated under acidic conditions (CD3CN/D20 10%/TFA-d 0.1%) after heating to 100°C for 4 h. Compound 19 shows no visible change in the 1H-NMR spectrum after 4 h. In panels (C) and (D), peaks labeled “S” are from D2O, p-xylene (internal standard for integration) and CD3CN.