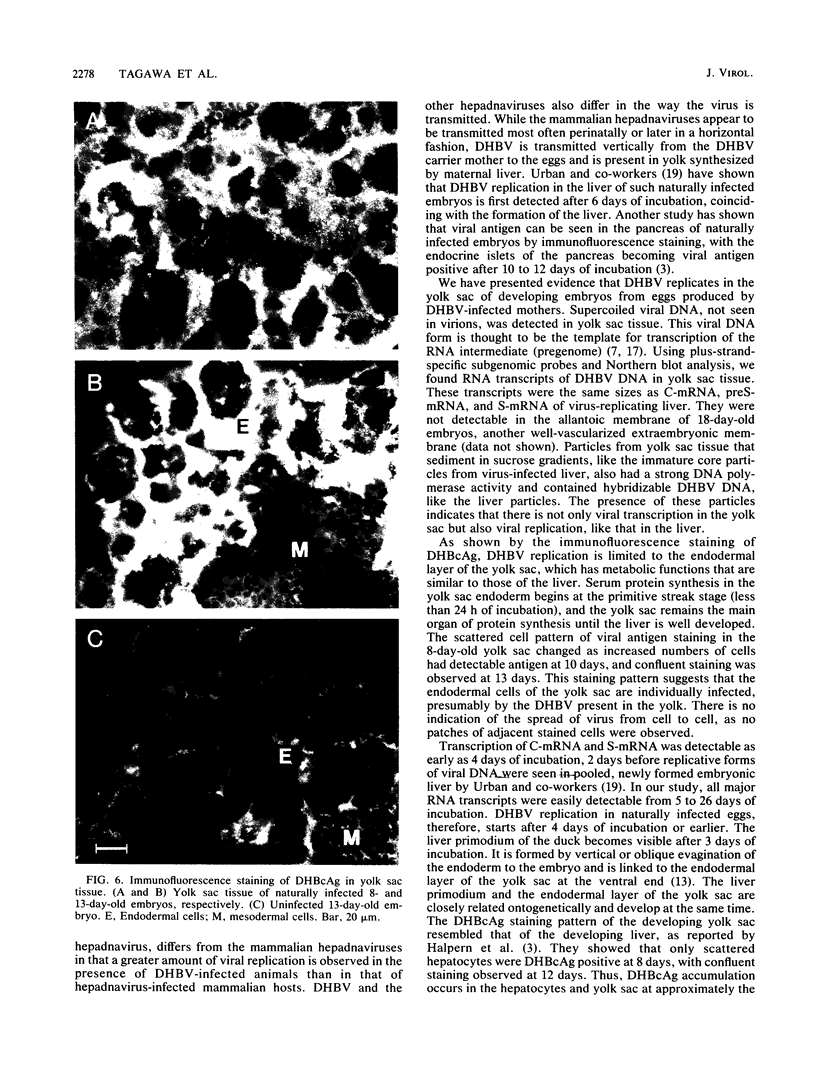
Abstract
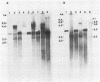
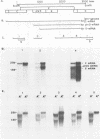
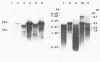
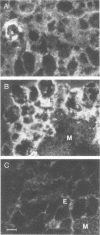
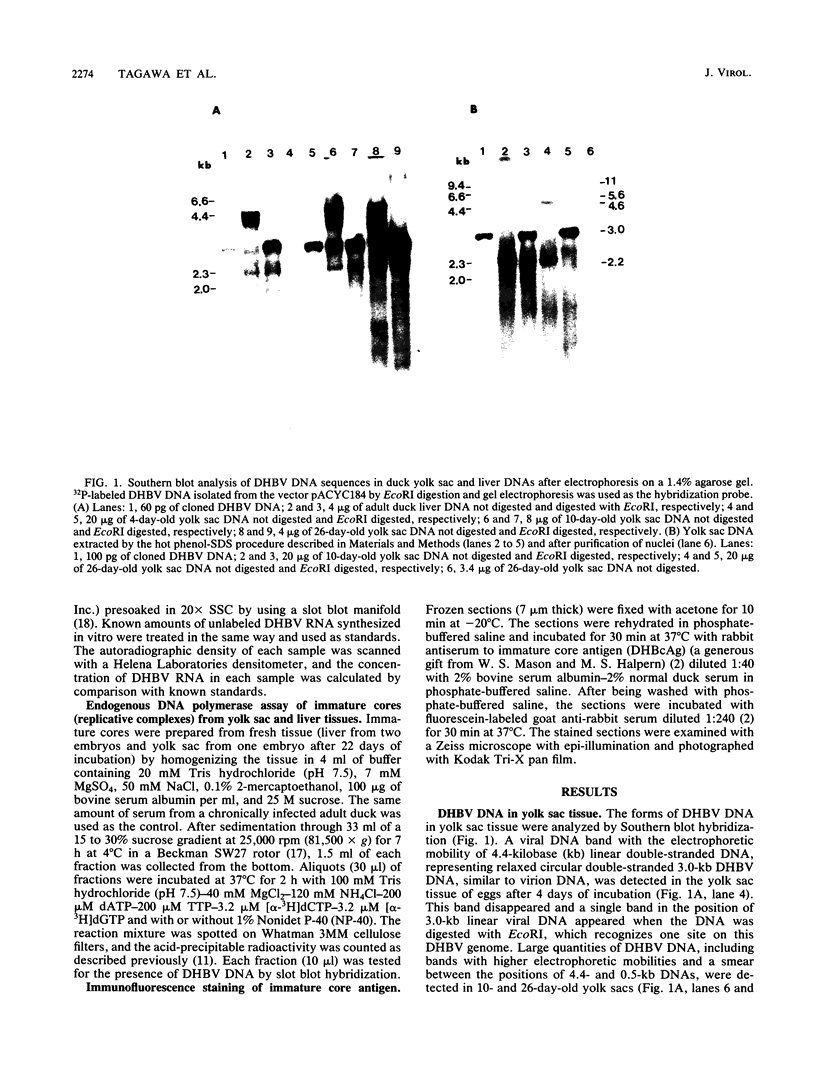
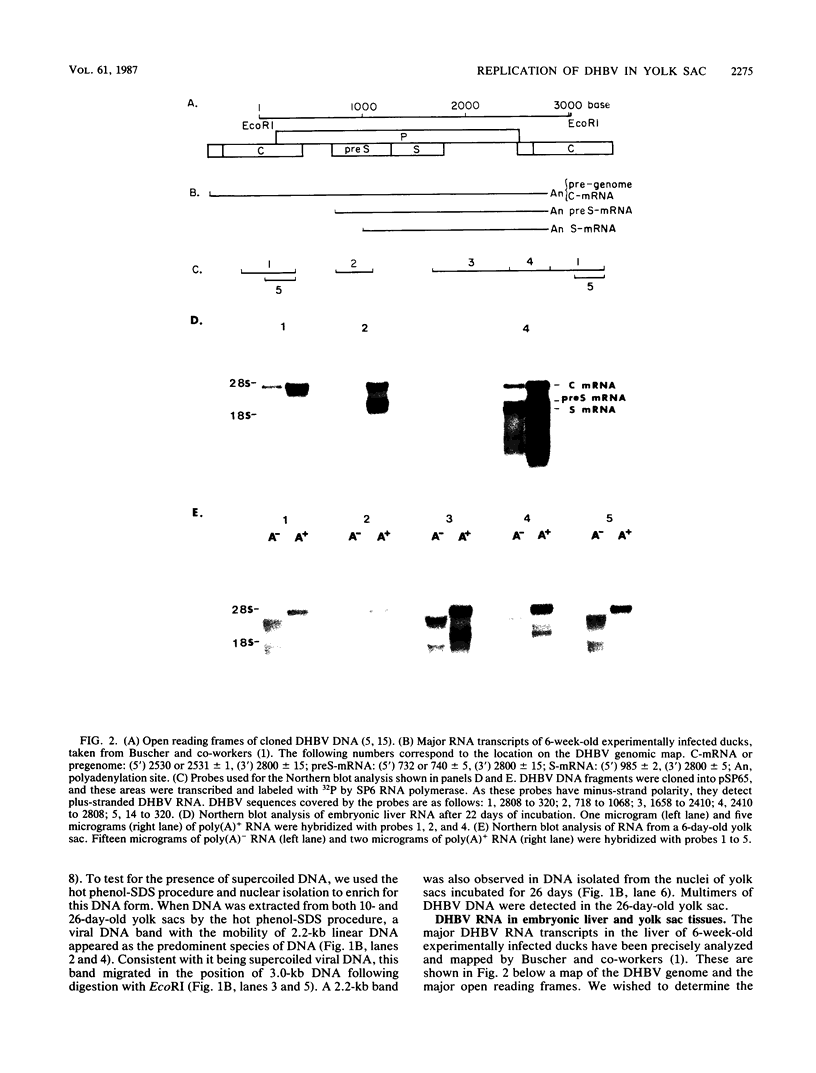
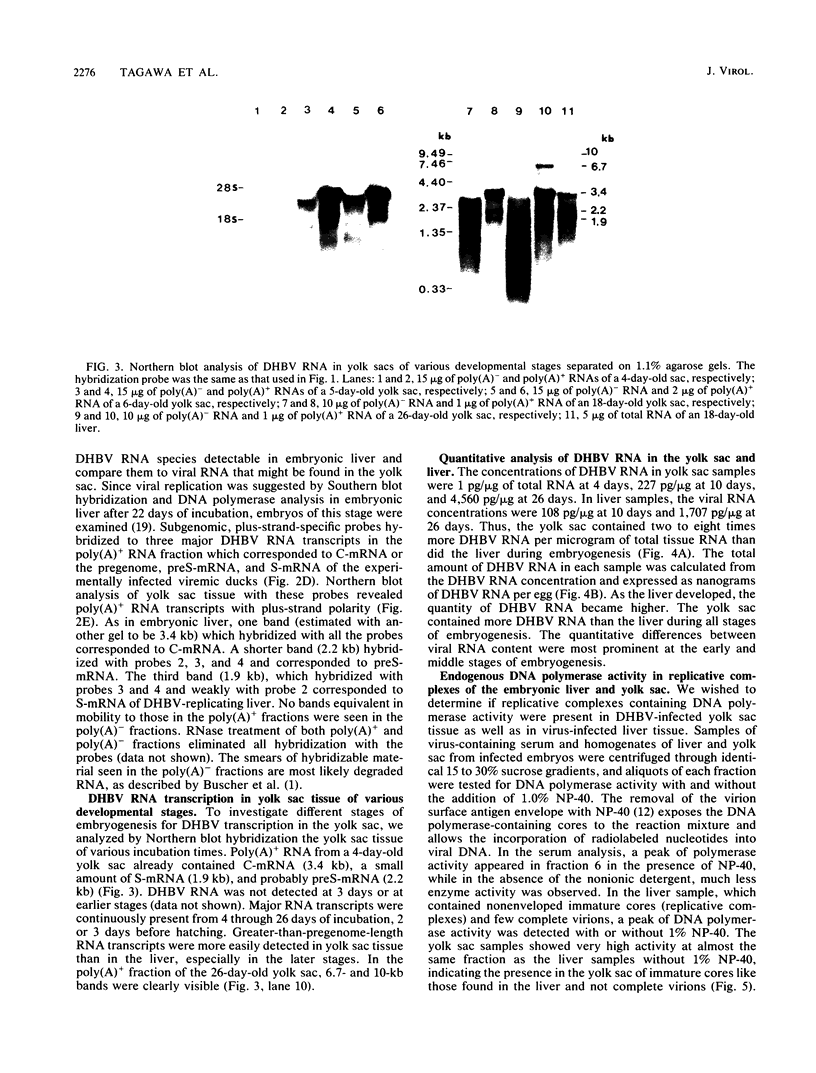
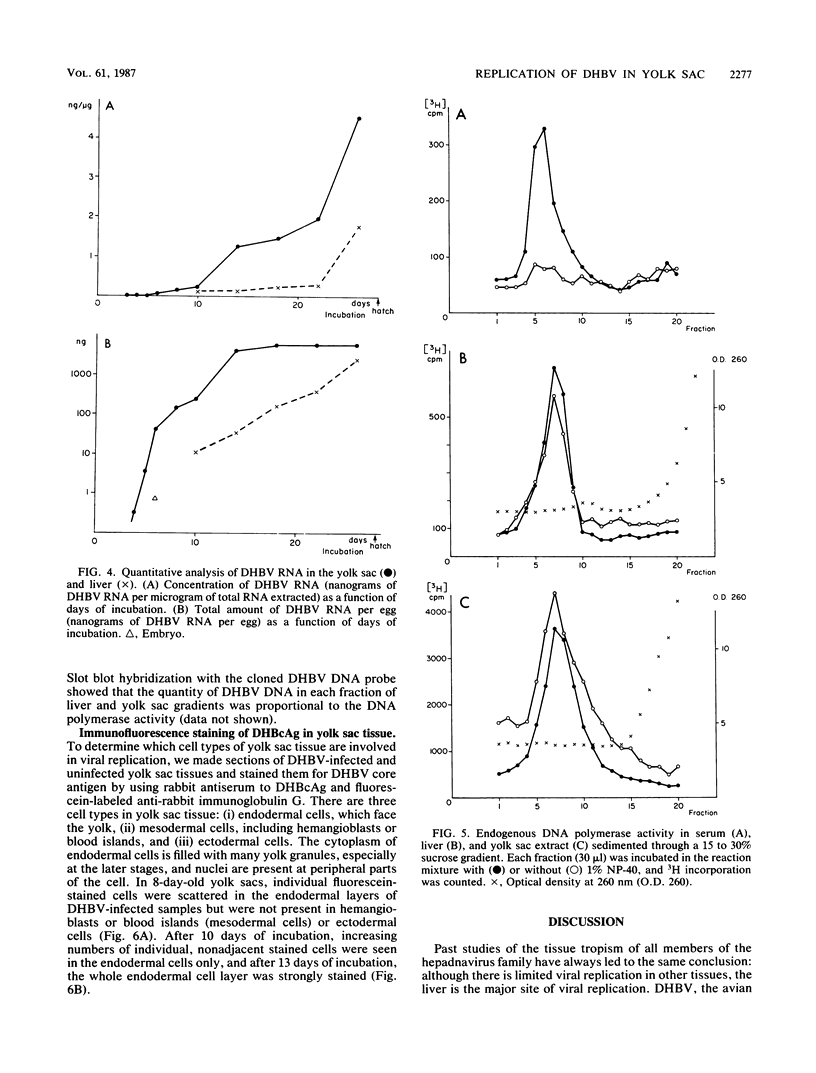
Duck hepatitis B virus (DHBV) is the only member of the hepadnavirus family in which nearly 100% vertical transmission from carrier mother to embryo has been reported. Large quantities of maternally transmitted virus particles are present in the yolk prior to incubation of the eggs, and replicative forms of DHBV DNA are detectable in the liver at 6 days of incubation. Since the yolk sac is similar to the liver in its production of serum proteins, we examined the yolk sacs of developing embryos for signs of viral replication. We detected the supercoiled form of DHBV DNA, DHBV RNA transcripts similar to those in the virus-replicating liver, and DNA polymerase activity and viral DNA in corelike particles in extracts of yolk sac tissue of naturally infected eggs. DHBV core antigen was strongly stained in only the endodermal layer of the yolk sac by immunofluorescence. DHBV RNA was detectable in the yolk sac from 4 days of incubation until hatching, and a larger quantity of DHBV RNA was present in the yolk sac than in the liver during all the stages of embryogenesis. Our data indicate that DHBV replicates actively in the yolk sac from an earlier stage than that previously reported in studies of embryonic liver and that replication is limited to the endodermal cell layer, which is ontogenetically and functionally related to the liver. The yolk sac may support the vertical transmission of DHBV.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Flores L., Egan J., Newbold J., Mason W. S. Individual cells in tissues of DHBV-infected ducks express antigens crossreactive with those on virus surface antigen particles and immature viral cores. Virology. 1984 Sep;137(2):408–413. doi: 10.1016/0042-6822(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., McMahon S. B., Mason W. S., O'Connell A. P. Viral antigen expression in the pancreas of DHBV-infected embryos and young ducks. Virology. 1986 Apr 15;150(1):276–282. doi: 10.1016/0042-6822(86)90288-6. [DOI] [PubMed] [Google Scholar]
- Kram D., Klein N. W. Serum protein synthesis in the early chick embryo. Dev Biol. 1976 Sep;52(2):300–309. doi: 10.1016/0012-1606(76)90247-5. [DOI] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Halpern M. S., England J. M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983 Dec;131(2):375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell A. P., Urban M. K., London W. T. Naturally occurring infection of Pekin duck embryos by duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1703–1706. doi: 10.1073/pnas.80.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. DNA and DNA polymerase in the core of the Dane particle of hepatitis B. Am J Med Sci. 1975 Jul-Aug;270(1):151–159. doi: 10.1097/00000441-197507000-00021. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Greenman R. L. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974 Jun;13(6):1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Chakraborty P. R., Shafritz D. A. Evidence for supercoiled hepatitis B virus DNA in chimpanzee liver and serum Dane particles: possible implications in persistent HBV infection. Cell. 1982 May;29(1):129–136. doi: 10.1016/0092-8674(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Kuhn C., Will H., Schaller H. Comparative sequence analysis of duck and human hepatitis B virus genomes. J Med Virol. 1985 Apr;15(4):323–333. doi: 10.1002/jmv.1890150402. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981 Mar-Apr;1(2):179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- Tagawa M., Omata M., Okuda K. Appearance of viral RNA transcripts in the early stage of duck hepatitis B virus infection. Virology. 1986 Jul 30;152(2):477–482. doi: 10.1016/0042-6822(86)90151-0. [DOI] [PubMed] [Google Scholar]
- Urban M. K., O'Connell A. P., London W. T. Sequence of events in natural infection of Pekin duck embryos with duck hepatitis B virus. J Virol. 1985 Jul;55(1):16–22. doi: 10.1128/jvi.55.1.16-22.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. F., Klein N. W. Synthesis of serum proteins by cultures of chick embryo yolk sac endodermal cells. Dev Biol. 1983 Nov;100(1):50–58. doi: 10.1016/0012-1606(83)90199-9. [DOI] [PubMed] [Google Scholar]
- Young M. F., Minghetti P. P., Klein N. W. Yolk sac endoderm: exclusive site of serum protein synthesis in the early chick embryo. Dev Biol. 1980 Mar;75(1):239–245. doi: 10.1016/0012-1606(80)90159-1. [DOI] [PubMed] [Google Scholar]