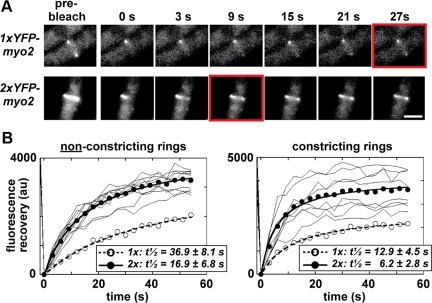
Figure 3.
Doubling Myo2p cellular levels increases its exchange rate at contractile rings. (A) FRAP was used to measure YFP-Myo2p exchange rates in contractile rings using confocal laser scanning fluorescence microscopy. Micrographs compare recovery of YFP-Myo2p fluorescence in nonconstricting rings from 1x (top panels) and 2xYFP-myo2 (bottom panels) cells. Panels on the far left show prebleached rings that were subsequently bleached at a region of interest (ROI). Subsequent panels chart recovery of signal at the ring (0–27 s after bleach). Red boxes indicate the point when recovery is half-maximal (t1/2). Cells were grown in YE5S media at 25°C before imaging at ambient temperature. White bar, 4 μm. (B) Plots charting FRAP at ROIs on nonconstricting and constricting rings from 2xYFP-myo2 cells. Fluorescence intensities measured before bleach (−1.5 s) and after bleach (every 3 s, 0–60 s) are plotted. Individual ROI traces (thin lines) are shown (n = 8–10) along with an average fit (•, thick line). Datasets for each trace were corrected for additional bleaching encountered during time-lapse imaging by a control ROI (derived from an unbleached ring in the same field of cells). To facilitate curve fitting, zero signal intensity was set for each trace by subtracting residual YFP-Myo2p signal (detected at the first time point after bleach, 0 s) from all trace values. Mean YFP-Myo2p recovery curves from FRAP experiments performed in parallel with 1xYFP-myo2 cells (Sladewski et al., 2009) are included on each plot (○, dashed line). t1/2 values represent the mean generated from the fits of each individual FRAP experiment.