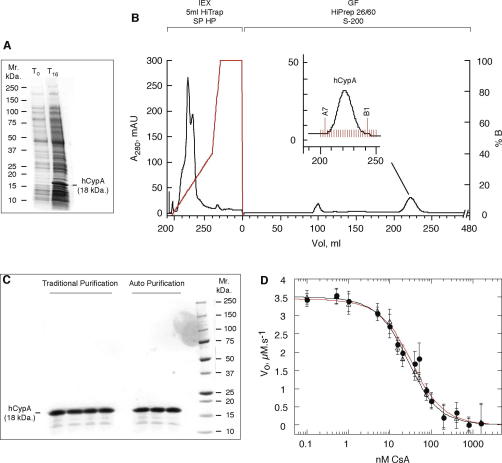
Fig. 1.
Automated purification of hCypA. (A) SDS–polyacrylamide gel (4–20% gradient) illustrating the typical levels of soluble hCypA over-expressed from OverExpress C41 BL21(DE3) E. coli grown for 16 h at 37 °C in Overnight Express Instant TB Medium. hCypA makes up ∼6% of the total soluble protein. To, soluble cell extract at mid log phase immediately after inoculation (A600 nm ≈ 0.5); T16, soluble cell extract following 16 h of growth shaking (260 rpm) at 37 °C. (B) Typical chromatogram for the automated 2-step purification of hCypA using ÄKTAXpress™. The pre-packed columns used are illustrated above the corresponding section of the chromatogram; IEX – ion-exchange, GF – gel-filtration. Solid black; A280 nm in mAU (left axis). Solid red; NaCl gradient in % Buffer-B (right axis). The inset details the region of the gel-filtration column elution from which fractions were collected. Indicated fractions A7–B1 were pooled. (C) SDS–polyacrylamide gel (4–20% gradient) illustrating the final purity levels of hCypA purified by both traditional and automated protocols. Both methods produce protein of ⩾93% purity (determined by gel densitometry). Five μg total protein was loaded in each lane. Four independent traditional runs and 3 independent automatic runs are shown, illustrating the excellent reproducibility of both methods and the excellent comparable purity between the methods. Molecular weight markers are shown to the right of the gel. (D) Inhibition of the PPIase activity (Vo in μM−1 s−1) of hCypA (15 nM) by cyclosporin (CsA). hCypA purified by either the traditional or automatic method shows the same high specific activity. Open triangles, black line, automatically purified hCypA; solid circles, red line, traditionally purified hCypA. The solid lines are a best fit to Eq. (1) (see Materials and methods). The values for the equilibrium dissociation constant for cyclosporin inhibition (Ki) are 24.3 ± 4.2 nM, for traditionally purified hCypA and, 19.7 ± 2.8 nM, for automatically purified hCypA, agreeing very well with literature values [42,44,48]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)