Figure 3.
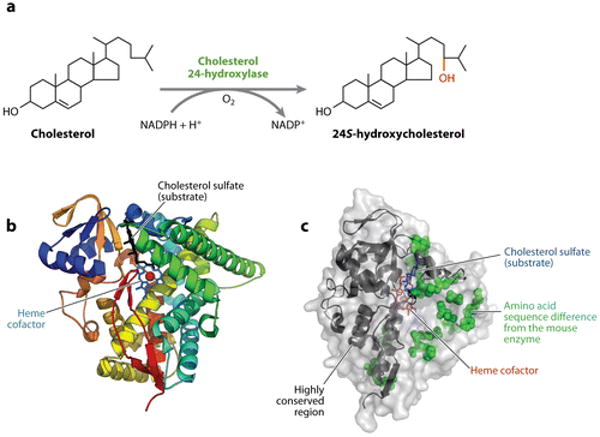
Cholesterol 24-hydroxylase. (a) The reaction catalyzed by cholesterol 24-hydroxylase. (b) The structure of the human enzyme as determined by X-ray crystallography. Twelve α-helices and four β-pleated sheets are shown in various colors together with the heme cofactor and a cholesterol sulfate substrate. Redrawn and reprinted with permission from Reference 53. (c) Surface representation of the human enzyme. Twenty amino acid sequence differences with the mouse enzyme, which are concentrated on one side of the protein, are shown as space-filling representations. Regions of the protein at the N and C termini that are more highly conserved are indicated by ribbon diagrams. Data extracted from Protein Data Bank entry 2q9f.
