Abstract
Leukemia-initiating cells (LICs) in acute myeloid leukemia (AML) are believed to be restricted to the CD34+ fraction. However, one of the most frequently mutated genes in AML is nucleophosmin (NPM), and this is associated with low CD34 expression. We, therefore, investigated whether NPM-mutated AMLs have LICs restricted to the CD34+ fraction. We transplanted sorted fractions of primary NPM-mutated AML into immunodeficient mice to establish which fractions initiate leukemia. Approximately one-half of cases had LICs exclusively within the CD34− fraction, whereas the CD34+ fraction contained normal multilineage hematopoietic repopulating cells. Most of the remaining cases had LICs in both CD34+ and CD34− fractions. When samples were sorted based on CD34 and CD38 expression, multiple fractions initiated leukemia in primary and secondary recipients. The data indicate that the phenotype of LICs is more heterogeneous than previously realized and can vary even within a single sample. This feature of LICs may make them particularly difficult to eradicate using therapies targeted against surface antigens.
Introduction
Normal hematopoiesis is organized as a hierarchy with hematopoietic stem cells (HSCs) at the apex and differentiated blood cells at the base. Acute myeloid leukemia (AML) is thought to be organized in a similar way, with leukemia-initiating cells (LICs) at the top of the hierarchy giving rise to variably differentiated blasts.1 LICs are thought to be critical to the growth of AML; hence, elimination of these is important to obtain a cure. To study and target LICs, one must know their phenotype. Two publications suggest that they all reside within the CD34+CD38− fraction of AML.1,2
We have recently shown that anti-CD38 antibody eliminates some LICs from immunodeficient mice through immune clearance.3 When this effect of anti-CD38 antibody was abrogated, we noted that the CD34+CD38+ fraction of 7 AML samples could initiate leukemia in immunodeficient mice. Moreover, we noted that some leukemias had no LICs in the CD34+CD38− fraction. This suggested that there is more heterogeneity in the phenotype of LICs than the original studies indicate.
Nucleophosmin (NPM) is one of the genes most commonly mutated in AML.4 NPM is a heterozygous mutation5 that appears to be stable over the disease course.6,7 AML with NPM mutation has distinct clinical,5,8,9 cytogenetic,4,10 molecular,11–13 and immunologic features4,7,13 that have led to its inclusion in the World Health Organization Classification of Tumors of the Hematopoietic and Lymphoid Tissues (4th edition) as a separate provisional entity.14 Of note, NPM-mutated AML is associated with low expression of CD34.4,7,13 Although many AML samples are termed “CD34−” by diagnostic laboratories, negativity is typically defined by less than 20% of blast cells expressing CD34. Thus, many samples that are defined as CD34− actually have a small population of CD34+ cells, and this may contain the LICs as previously reported. However, we have observed normal human hematopoietic cells in the bone marrow of mice transplanted with the CD34+CD38− fraction of one AML with mutated NPM, suggesting that this fraction may contain normal hematopoietic progenitors in some AML specimens.3 This led us to question whether LICs express CD34 in all NPM-mutated AML specimens.
To investigate this, we tested CD34+ and CD34− cells from NPM-mutated AMLs using genomic and functional assays. CD34+ and CD34− cells were transplanted into immunodeficient mice to identify which fraction contained LICs. We used the most immunodeficient mice available and used measures to abrogate the effect of antibody-mediated clearance.3 The CD34− cells alone initiated leukemia from approximately half of samples (that we termed subtype A), whereas the CD34+ cells gave rise to normal multilineage hematopoiesis. Most other samples had LICs within both CD34+ and CD34− fractions, and these were capable of transmitting leukemia to secondary recipients. The data indicate that one cannot blindly rely on the CD34+CD38− phenotype to identify LICs from all AMLs. We also show that the phenotype of LICs can change and this provides a probable explanation for the presence of LICs in multiple fractions from some AMLs.
Methods
Primary cells
Cord blood and AML cells were obtained after informed consent at St Bartholomew's and the Royal London Hospitals. The protocol was approved by the East London and City Research Ethics Committee. The samples were collected at untreated presentation (n = 26) or relapse (n = 1). The median white blood count was 103 (range, 12-313) × 109 cells per liter. Details of the patient samples are listed (Table 1). Peripheral blood was used as a source of AML cells.
Table 1.
Characteristics of AML patient samples
| AML sample | FAB | NPM mutation | FLT3 | Karyotype | Total CD34+ % | Subtype |
|---|---|---|---|---|---|---|
| 1 | M1 | TCTG | ITD | Normal | 0.14 | A |
| 2 | M1 | TCTG | WT | Normal | 0.05 | A |
| 3 | M2 | TCTG | WT | Normal | 0.05 | A |
| 4 | M4 | TCTG | WT | Normal | 0.15 | A |
| 5 | M4 | TCTG | ITD | 47,XX,+13 | 0.1 | A |
| 6 | M4 | TCTG | WT | Normal | 0.12 | A |
| 7 | M5 | TCTG | WT | 47,XX,+der(?)t(1;?)(q21;?) | 0.19 | A |
| 8 | M1 | CCTG | WT | Failed | 0.13 | A |
| 9 | M1 | TCTG | ITD | Normal | 0.28 | A |
| 10 | M2 | CATG | WT | 46,XX,del(11)(p11.2p13)[7]; 46,idem,t(2;3)(q33;p11∼p13)[3] | 0.06 | A |
| 11 | M2 | TCTG | ITD | Normal | 0.06 | A |
| 12 | M5 | TCTG | WT | Normal | 0.25 | A |
| 13 | M5 | TCTG | WT | Normal | 0.085 | A |
| 14 | M4 | TCTG | WT | 48,XY,+3 +10 | 5.5 | B |
| 15 | M5 | TCTG | WT | Normal | 3.1 | B |
| 16 | M5 | TCTG | ITD | Normal | 0.66 | B |
| 17 | M1 | CTTG | ITD | Normal | 0.85 | C |
| 18 | M4 | TCTG | ITD | Normal | 8.0 | C |
| 19 | M4 | CCTG | ITD | Normal | 11.1 | C |
| 20 | M1 | TCTG | NT | Normal | 53.2 | C |
| 21 | M1 | TCTG | WT | 47,XY,+13 | 3.3 | C |
| 22 | M4 | CCAG | ITD | Normal | 3.7 | C |
| 23 | M2 | CCTG | ITD | Normal | 1.9 | C |
| 24 | NT | TCTG | ITD | Normal | 26.9 | C |
| 25 | M1 | TCTG | ITD | Normal | 65.3 | C |
| 26 | M2 | CCTG | ITD | Normal | 8.6 | C |
| 27 | M1 | TCTG | WT | Normal | 2.4 | C |
The subtype refers to the classification based on the pattern of expression of CD34 and CD38. All samples except samples 25 and 26 have wild-type Wilms tumor 1 exons 7 and 9 (sample 18 was not tested).
FAB indicates French-American-British classification; NPM, nucleophosmin; FLT3, FMS-like tyrosine kinase; WT, wild type; ITD, internal tandem duplication; T, thymine; C, cytosine; G, guanine; and A, adenine.
Mutation analysis
NPM,5 FMS-like tyrosine kinase 3 (FLT3),15 and Wilms tumor 116 mutation sequencing was performed using previously published methods.
Immunophenotyping and cell sorting
All antibodies were obtained from BD Biosciences. AML cells were stained with phycoerythrin (PE)–conjugated anti-CD34, fluorescein isothiocyanate (FITC)–conjugated anti-CD38, and allophycocyanin (APC)–conjugated anti-CD3 antibody before resuspension in a 4,6 diamidino-2-phenylindole (DAPI) containing solution of 2% fetal calf serum (FCS) with phosphate buffered solution (PBS). Analysis was performed on a BD LSR2 or a BD Aria. Gates were set up to exclude nonviable cells and debris. Cell sorting was performed on a BD Aria after staining the cells as described above. For sorting cells pretransplantation CD3+ cells were excluded (none of the samples expressed CD3 on the blasts). Purity checks were performed to ensure sort quality. The purity of the CD34−CD38+ fraction and the CD34−CD38− fraction was 97.1% plus or minus 1.9% and 97.2% plus or minus 3.7%, respectively. The purity of the CD34+CD38− fraction and the CD34+CD38+ fractions were 92.8% plus or minus 6.2% and 91.4% plus or minus 3.8%, respectively.
Real-time quantitative polymerase chain reaction assay for NPM exon 12
DNA was extracted from 560 to 50 000 sorted cells using a QIAamp DNA mini kit (Qiagen) according to the manufacturer's instructions with minor modifications as reported previously.17 To improve DNA yield, sorted cells were centrifuged at 3800g. Real time quantitative analysis of NPM exon 12 mutations was done using previously published methods.18 The total amount of DNA present was determined by quantitation of Albumin (TaqMan Control Genomic DNA; Applied Biosystems). All samples were tested in triplicate. Standard curves for NPM and albumin were established by amplifying a serial dilution of NPM mutants from 50 000 to 5 cells per reaction. A standard curve was created with each run (supplemental Figure 1; available on the Blood website; see the Supplemental Materials link at the top of the page of the online article). The assay was able to detect 5 cells reliably. The percentage of mutated NPM was determined by dividing the value for NPM mutation by the albumin value. Percentages greater than 100% were treated as 100%.
Immunomagnetic depletion and enrichment of CD34+ cells
Easysep Human CD34 Selection Cocktail and Easysep magnet (StemCell Technologies) were used according to the manufacturer's instructions to enrich CD34+ cells from AML samples. The procedure resulted in an increase in percentage of CD34+ cells by more than 30-fold. Unbound CD34 depleted cells were obtained from the residual supernatant after CD34 enrichment. This depleted 88% plus or minus 8% of the CD34+ cells in the R1 gate (see Figure 2Ai) from subtype A samples and 97% of the CD34+CD38− cells from one subtype B sample.
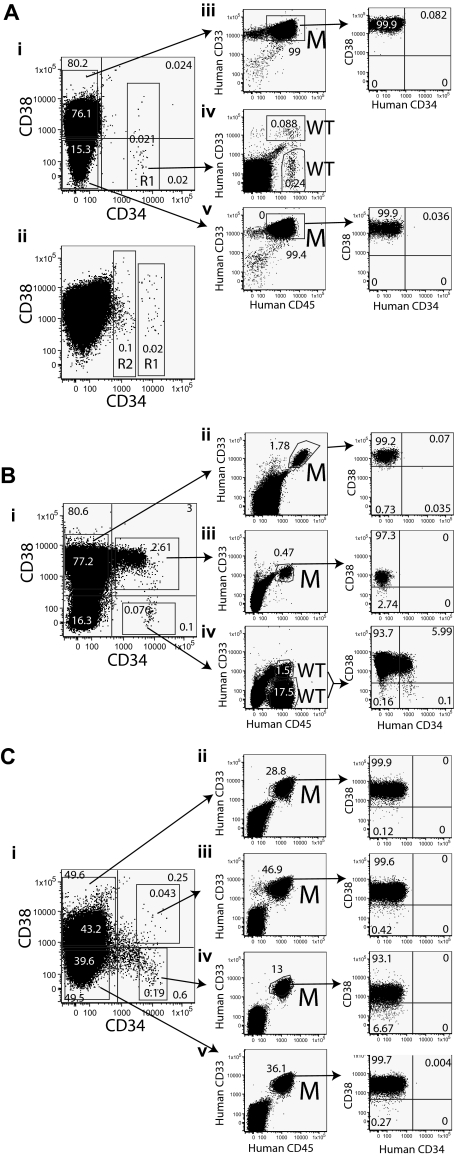
Figure 2.
Sorting strategy and results of transplantation from fractions of NPM mutant AML. The phenotypes and sorting strategies are displayed for subtypes A-C. Sorted fractions of the leukemias were transplanted into mice. The expression of human CD45, CD33, CD38, and CD34 on bone marrow cells from the mice is displayed in the plots on the right. The nature of the human cells was assessed by quantitative PCR, and the results are indicated by letters adjacent to the plots. M indicates mutated NPM and WT indicates wild-type NPM. (A) The phenotypes of 2 subtype A samples are displayed. The R1 region indicates the gate used for sorting CD34+ cells (Ai). Some subtype A samples also have a separate CD34dim population that is marked by the R2 region (Aii). AML was initiated by the CD34−CD38+ (Aiii) and CD34−CD38− (Av) fractions of sample 3 (subtype A), whereas CD34+CD38− cells (Aiv) initiated normal multilineage hematopoiesis. (B) Sample 15 (subtype B) was sorted as indicated (Bi). The CD34−CD38+ (Bii) and CD34+CD38+ (Biii) fractions of sample 15 initiated leukemia, whereas CD34+CD38− cells (Biv) initiated normal multilineage hematopoiesis. (C) Sample 17 (subtype C) was sorted as indicated (Ci). All fractions of sample 17 initiated AML (Cii-v) though both normal and leukemic cells arose from the CD34+CD38− fraction in some mice.
Colony-forming assays
Two to 500 × 103 cells from the CD34-enriched and CD34-depleted fractions were plated in triplicate in 1 mL of MethoCult GF+ (StemCell Technologies) in 35-mm tissue culture dishes. On day 14 of culture, the numbers of colonies were scored using an inverted microscope. Cells were harvested and washed twice with PBS 2% FCS before analysis by quantitative polymerase chain reaction (PCR).
Mice
Nonobese diabetic/severe combined immunodeficiency disease/β2-microglobulin–null (NOD/SCID/β2m−/−) and NOD/SCID/interleukin-2 receptor γ chain–null (NOD/SCID/IL2rγ−/−) mice were a kind gift of Dr Leonard Shultz (The Jackson Laboratory) and were used as detailed previously.19,20 All animal experiments were performed in accordance to Home Office and CRUK guidelines. To abrogate antibody-mediated clearance of cells, all mice received a total of 1 mg/g of human immunoglobulin (IVIG; Bio Products Laboratory) as described before.3 Mice received a sublethal dose of radiation (330-375 cGy) from a 137cesium source 24 hours before transplantation. Direct intra–bone marrow injection (as previously described21) was the preferred route of administration unless more than 106 cells were administered, for which the intravenous route was preferred.
Assessment of engraftment
Engraftment was assessed by immunophenotyping as described before.19 Briefly, normal multilineage engraftment was defined by the presence of separate CD45+CD33+ and CD45+CD19+ populations with the appropriate scatter characteristics. AML engraftment was defined by the presence of a single CD45+CD33+ population greater than 0.1% of live cells. The phenotype of engrafted cells was determined by staining bone marrow with Peridinin-chlorophyll protein (PerCP)–conjugated anti-CD45, PE-Cyanin 7 (PE-Cy7)–conjugated anti-CD14, APC-conjugated anti-CD15, and PE-conjugated anti-CD36 antibodies. In addition, the percentage of NPM-mutated cells in the graft was determined by quantitative genomic PCR. This was performed on sorted or unsorted cells from the bone marrow (there was no statistically significant difference between the approaches). We performed the assay on bone marrow cells (n = 15) from nontransplanted mice to determine nonspecific amplification and defined negativity based on this.
AML samples were screened for ability to generate a graft in immunodeficient mice. Only those samples capable of generating a leukemic graft were used in experiments to determine the phenotype of LICs; 21 of 25 NPM mutant AMLs were capable of engrafting immunodeficient mice.
Serial transplantation
Bone marrow cells from mice transplanted with fractions of AML were stained with APC-conjugated anti–mouse CD45 antibody and PE-conjugated anti–human CD33 antibody before resuspension in a DAPI-containing solution of 2% FCS with PBS. Human CD33+ cells were sorted on a BD Biosciences FACSAria before transplantation into irradiated mice. In some experiments, particularly where engraftment of leukemia was high, bone marrow cells were transplanted without cell sorting.
Statistics
Results are expressed as mean with standard deviation unless stated. The Student t test was used to assess the significance of any differences. The chi-square test was used to assess the significance of differences between FLT3 mutation frequency in different subgroups. Extreme Limiting Dilution Analysis software (available from the Bioinformatics section of the Walter and Eliza Hall Institute of Medical Research, http://bioinf.wehi.edu.au/software/elda/index.html) was used to estimate the frequency of LICs from limiting dilution assays and differences between fractions.22
Results
Classification of AML with NPM mutation according to phenotype
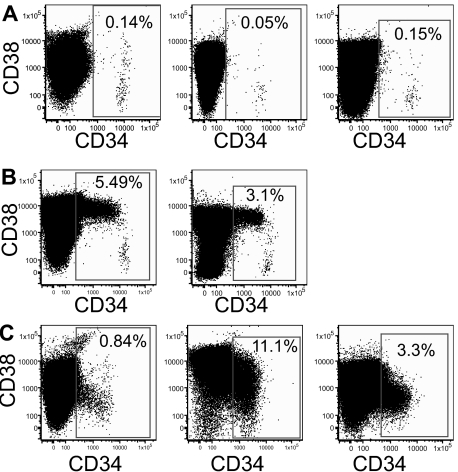
Immunophenotyping was performed on 27 AML samples, all of which had mutated NPM (Table 1). The median CD34+ expression was 0.66% (range, 0.05%-65.3%), and this was significantly lower (P < .001) than that seen in 30 AMLs with wild-type NPM (median CD34+ expression was 24.5%; range, 0.07%-90%; data not shown). The NPM-mutated leukemias were classified into 3 distinct subtypes based on their CD34 and CD38 expression. Subtype A samples (13 of 27 AMLs) were predominantly CD34−, with a total CD34+ less than 0.5% (Figure 1A). Subtype B and C samples have a total CD34+ greater than 0.5%. The subtype B samples (3 of 27 samples) have a small CD34+CD38− population (< 0.1% of total) that is distinct from the CD34− and CD34+CD38+ populations (Figure 1B). Although the subtype C samples (11 of 27 samples) may have a small CD34+CD38− population, it is never separated clearly from the other fractions (Figure 1C). The subtype C samples had a higher incidence of FLT3 ITD than subtype A samples (80% vs 31%, respectively; P = .02).
Figure 1.
Classification of NPM mutant AML samples. The expression of CD34 and CD38 on 8 AML samples is shown. (A) Subtype A samples have CD34 expression less than 0.5% of cells. The phenotypes of 3 subtype A samples are shown (left, sample 1; middle, sample 3; and right, sample 4). (B) Subtype B samples have CD34 expression greater than 0.5%, but the CD34+CD38− population is small (< 0.1%) and distinct from the CD34+CD38+ and CD34− populations. The phenotypes of samples 14 (left) and 15 (right) are shown. (C) Subtype C samples are more heterogeneous than the other subtypes. The CD34+ fraction is greater than 0.5%, but there is no distinct and small CD34+CD38− population. The phenotypes of samples 17 (left), 19 (middle), and 21 (right) are displayed.
The majority of cells within the CD34+CD38− fraction of subtypes A and B do not contain mutated NPM
Earlier studies suggested LICs are enriched within the CD34+CD38− fraction of AML. To investigate whether CD34+CD38− cells from NPM-mutated AML are leukemic or normal, we determined the percentage of mutated NPM in DNA from sorted cells from 12 AML samples (supplemental Figure 1). Four cell fractions were tested (CD34+CD38−, CD34+CD38+, CD34−CD38+, and CD3+). The CD3+ cells and CD34−CD38+ cells (this latter population comprises the majority of cells from most of the AML samples) served as negative and positive controls, respectively. We used the region R1 in Figure 2Ai to sort CD34+CD38− and CD34+CD38+ fractions from 8 subtype A samples. We also tested the distinct CD34dim population of cells (region R2 in Figure 2Aii) found in 5 of 13 subtype A samples. The data summarized in Table 2 suggest that the majority of cells in the defined CD34+CD38− and CD34+CD38+ fractions of pattern A samples contain wild-type NPM. There was no significant difference between the percentage of mutated DNA in the CD3+ cells and the CD34+CD38− and CD34+CD38+ fractions from subtype A samples. Given that the T lymphocytes are thought to be genetically normal in NPM-mutated AML,23 we interpret the finding of small amounts of mutated DNA in these 3 populations as being indicative of low-level contamination of each population by leukemic cells during sorting; 100% (sample 8) and 76% (sample 9) of cells in the CD34dim population contained mutant NPM. All fractions except the CD3+ from one subtype C sample contained mostly mutated NPM.
Table 2.
Percentage of NPM-mutated cells within sorted fractions of AML
| Sample | Subtype | CD34+CD38− | CD34+CD38+ | CD3+ | CD34−CD38+ |
|---|---|---|---|---|---|
| 1 | A | 5 | 10.4 | 3.5 | 99 |
| 2 | A | 1.7 | 0 | 3.8 | 100 |
| 3 | A | 7.5 | 14 | 0 | 90.5 |
| 4 | A | 3.9 | 60 | 0 | 100 |
| 5 | A | 0 | 1.2 | 8.6 | 100 |
| 6 | A | 0 | 13.8 | 0.2 | 97 |
| 8 | A | 7.3 | 16 | 2.4 | 97 |
| 9 | A | 7.7 | 5 | 2.4 | 100 |
| Mean of subtype A | 4.1 ± 3.3* | 15 ± 19.1 | 2.6 ± 2.9† | 97.9 ± 3.3 | |
| 14 | B | 12.2 | 83 | 1.4 | 100 |
| 15 | B | 16.5 | 100 | ND | 94.9 |
| 16 | B | 7.4 | 45 | 1.4 | 100 |
| Mean of subtype B | 12 ± 4.6* | 76 ± 28.2 | 1.4 | 98.3 ± 2.0 | |
| 20 | C | 94 | 98.7 | 0.3 | 100 |
The AML sample number is in the first column. The figures are percentages of cells that contain mutated NPM derived from quantitative genomic PCR. ND indicates not done.
The percentage of NPM-mutated cells in the CD34+CD38− cells is significantly lower than in the CD34−CD38+ fraction (P < .001 for subtypes A and B).
There is no significant difference between the percentage of NPM in the CD3+ fraction and the CD34+CD38− (P = .3) or in the CD34+CD38+ (P = .1) fractions of subtype A samples.
The CD34+ fraction from subtype A samples contain all the erythroid colony–forming cells
To provide additional evidence that many CD34+ cells are normal in NPM-mutated AML, we determined whether CD34+ cells produce normal colonies in methylcellulose. CD34+ cells were enriched from 6 AML samples (4 subtype A [samples 1, 3, 8, and 9] and 2 other subtype AMLs [samples 14 and 17]) by immunomagnetic positive selection and plated into methylcellulose. The CD34 enriched cells gave rise to erythroid colonies from all samples (supplemental Figure 2). By contrast, samples depleted of CD34+ cells did not produce erythroid colonies in any of the plates (supplemental Figure 2). The frequency of erythroid colonies varied from 9.5 to 138 colonies per 105 cells. The percentage of mutant NPM was determined in the cells from the methylcellulose. The mean percentage of NPM-mutated cells was significantly lower (P = .003) in the cells derived from the CD34-enriched fraction (12.3% ± 17%) than from the CD34-depleted fraction (75.3% ± 36%). This experiment shows that normal progenitors are found in the CD34+ fraction of these leukemias. Given that 4 of these samples had very small CD34+ fractions (< 0.3% of total cells), the data provide additional evidence that the CD34+ cells are normal.
The CD34− fraction of subtype A samples gives rise to AML, whereas the CD34+ fraction gives rise to normal hematopoiesis
Although most of the CD34+ cells from subtype A samples contained wild-type NPM, the minority that contain mutated NPM may be LICs. To test this, we transplanted NOD/SCID/β2m−/− mice with cells from the CD34−, CD34+, or CD34dim (from the R2 region in Figure 2Aii) fractions from 5 subtype A samples. CD34− cells (derived from cell sorting or immunomagnetic depletion) were transplanted and gave rise to leukemia (Table 3). By contrast sorted CD34+ cells and CD34dim cells failed to initiate leukemia. The CD34+ fraction from 2 samples gave rise to normal multilineage hematopoiesis. The data indicate that LICs are found in the CD34− fraction of subtype A samples.
Table 3.
Results of transplantations from CD34−, CD34dim, and CD34+ fractions of AML into immunodeficient mice
| Sample | Time, weeks | CD34− |
CD34+ |
CD34dim |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose × 106 | MNC dose × 106 | Mice with AML/mice total | Dose × 103 | MNC dose × 106 | Mice with AML/mice total | Dose × 103 | MNC dose × 106 | Mice with AML/mice total | ||
| 1 | 12-13 | 24 | 24 | 2/2* (2.8%) | 7.8 | 5.5 | 0/3† | NA | NA | NA |
| 1 | 12 | 5 | 5 | 0/2 | ||||||
| 2 | 12 | 21 | 21 | 2/2* (1.2%) | NT | NT | NT | NA | NA | NA |
| 7 | 9 | ‡ | ‡ | ‡ | 2.5 | 5.2 | 0/1 | 11 | 9.2 | 0/1 |
| 8 | 12 | 5.4 | 5.4 | 2/3* (0.1%) | 4.2 | 19 | 0/1† | 5 | 5.2 | 0/2 |
| 9 | 13-15 | 13 | 13 | 4/4* (68%) | 8.4 | 15 | 0/1 | 400 | 64 | 0/1 |
| WT1 | 9 | 6 | 6 | 1/1 (60%) | 3.2 | 6 | 0/1 | NA | NA | NA |
NA indicates not applicable as these samples lack the CD34dim population; NT, not tested; and WT1, wild-type NPM AML sample 1. The figures in parentheses indicate the mean percentage of AML in the mouse bone marrow.
Sorted human cells from mouse bone marrow were shown to contain mutated NPM by quantitative PCR.
Normal multilineage engraftment observed; the DNA from the grafts contained wild-type NPM.
The LICs from sample 7 are found in the CD34− fraction (Table 4).
LICs are found within the CD34−CD38+ fraction of NPM samples but not the CD34+CD38− fraction of subtypes A and B
To determine the expression of CD38 and CD34 on LICs from NPM-mutated AML, we transplanted mice with sorted cells from the CD34−CD38−, CD34−CD38+, CD34+CD38+, and CD34+CD38− fractions (Table 4 and supplemental Tables 1-2). To confirm the nature of the human CD45+ grafts, we performed quantitative PCR on engrafted cells from the majority of experiments. The CD34−CD38+ fraction initiated leukemia from all 12 NPM-mutated AML samples where this fraction was tested, whereas the CD34−CD38− fraction did from 6 of 12 samples. For subtypes A and B, the CD34+CD38− fraction gave rise to normal multilineage hematopoiesis (the engrafted cells contained wild-type NPM) or no graft. The CD34+CD38− fraction gave rise to leukemia from 5 subtype C samples, though some mice contained both AML and normal human hematopoiesis.
Table 4.
Results of the transplantation of CD34−CD38−, CD34−CD38+, CD34+CD38+, and CD34+CD38− fractions from AML
| AML sample | Subtype | Strain | Time, weeks | CD34−CD38− |
CD34−CD38+ |
CD34+CD38+ |
CD34+CD38− |
|---|---|---|---|---|---|---|---|
| Mice with AML/mice transplanted (mean percentage of AML) | Mice with AML/mice transplanted (mean percentage of AML) | Mice with AML/mice transplanted (mean percentage of AML) | Mice with AML/mice transplanted (mean percentage of AML) | ||||
| 1 | A | B2m | 12 | NT* | NT* | NT* | 0/2† |
| 3 | A | NSG | 12-14 | 2/2‡ (99%) | 3/3‡ (49%) | 0/1 | 0/2† |
| 7 | A | B2m | 5-9 | 0/2 | 3/3‡ (70%) | NT§ | NT§ |
| 9i | A | NSG | 17 | 2/2‡ (53%) | 1/1‡ (84%) | 0/1 | Died |
| 9ii | A | B2m | 12-15 | 1/2‡ (0.1%) | 1/1 (0.3%) | NT | 0/1† |
| 10 | A | NSG | 16 | 1/1 (0.3%) | 2/2‡ (26%) | 0/1 | 0/1 |
| 12 | B | NSG | 16 | 0/2 | 2/2‡ (0.3%) | 0/1 | 0/3† |
| 15 | B | NSG | 8-15 | 0/1 | 1/3‡ (2.5%) | 4/5‡ (1.4%) | 0/3† |
| 17i | C | B2m | 9-12 | 6/6‡ (57%) | 7/8‡ (39%) | 6/8‡ (32%) | 4/6‖ (38%) |
| 17ii | C | NSG | 12 | NT | NT | NT | 1/2‖ (76%) |
| 18 | C | B2m | 10 | 0/1 | 1/1‡ (4.4%) | 1/1 (3.7%) | 1/1‖ (1.6%) |
| 19 | C | B2m | 12 | 0/2 | 1/2‡ (0.1%) | 0/2 | 1/2‡ (0.3%) |
| 24 | C | B2m | 12 | 1/1 (20%) | 2/2 (40%) | 2/2 (11%) | (24%) |
| 25 | C | B2m | 12 | 0/2 | 3/3 (6.7%) | 3/3 (26%) | 0/1 |
| 26 | C | B2m | 12 | 3/3 (73.3%) | 3/3 (37.2%) | 1/1 (8.9%) | 1/1 (0.9%) |
| WT2 | NA | B2m | 7 | 4/4 (49%) | 0/1 | 0/1 | NA |
The AML sample is indicated in the first column and the subtype in the second. Some samples were tested in more than one strain of mouse (indicated by the lowercase Roman numeral). NSG indicates that NOD/SCID/IL2rγ−/− mice were used in the experiment; B2m, NOD/SCID/β2m−/− mice; NT, not tested; WT2, wild-type NPM AML sample 2; and NA, not applicable as this sample lacks a CD34+CD38− population. The figures in parentheses indicate the mean percentage of AML in the mouse bone marrow.
The LICs from sample 1 are restricted to the CD34− fraction (Table 3).
Normal multilineage engraftment observed from the CD34+CD38− fraction from one or more mice. The DNA from the grafts contained wild-type NPM.
The graft was assessed by quantitative PCR and shown to contain mutated NPM.
LICs are not found in the CD34dim and CD34+ fractions that include all the CD34+CD38+ and CD34+CD38− cells (Table 3).
Both CD45+CD33+ and CD45+CD19+ populations were observed in some mice transplanted with CD34+CD38− cells. The CD19+ cells contain mostly wild-type NPM, whereas the CD33+ fractions contain mostly mutant NPM, consistent with the presence of LICs and normal repopulating cells in the CD34+CD38− fraction. Some of the data from the CD34+CD38+ and CD34+CD38− fractions of samples 15 and 18 have been previously published.3
Serial transplantation
AML cells from successful grafts were transplanted into secondary recipients to assess self-renewal ability. Cells derived from the CD34−CD38+ and CD34−CD38− fractions of subtype A samples were capable of initiating leukemia in secondary recipients (Table 5). Cells from one secondary recipient were able to initiate leukemia in multiple tertiary recipients demonstrating self-renewal.
Table 5.
Details of secondary transplants
| Sample | Fraction | Secondary recipients* | Tertiary recipients* | Time in vivo, weeks† |
|---|---|---|---|---|
| 3 | CD34−CD38+ | 4/4 | 5/5 | 31 |
| 3 | CD34−CD38− | 2/2 | NT | 26 |
| 7 | CD34−CD38+ | 5/5 | 5/5 | 12 |
| 9 | CD34−CD38+ | 3/3 | NT | 34 |
| 9 | CD34−CD38− | 1/1 | NT | 34 |
| 17 | CD34−CD38+ | 3/3 | NT | 24 |
| 17 | CD34−CD38− | 3/3 | NT | 24 |
| 17 | CD34+CD38+ | 3/3 | NT | 18 |
| 17 | CD34+CD38− | 1/1 | NT | 25 |
| 26 | CD34−CD38+ | 3/3 | NT | 24 |
| 26 | CD34−CD38− | 4/4 | NT | 24 |
| 26 | CD34+CD38+ | 4/4 | NT | 24 |
| 26 | CD34+CD38− | 0/1 | NT | 24 |
| 24 | CD34−CD38+ | 1/1 | NT | 24 |
| 24 | CD34−CD38− | Mouse died | NT | 24 |
| 24 | CD34+CD38+ | 1/1 | NT | 24 |
| 24 | CD34+CD38− | 1/1 | NT | 24 |
NT indicates not tested.
Data for secondary and tertiary recipients are reported as mice with AML/mice transplanted.
Time in vivo refers to the time from transplantation in the primary recipient to the termination of the final recipient. Sample 7 is very aggressive clinically and mice became sick due to leukemia 3 to 5 weeks after transplantation, hence the relatively short duration.
Cells from mice transplanted with either CD34+ or CD34− fractions from 3 subtype C samples (samples 17, 24, and 26) were able to initiate leukemia in secondary recipients, indicating that both the CD34+ and CD34− LICs have self-renewal capacity (Table 5). Therefore, the LICs we describe can maintain and propagate leukemia over prolonged periods and are not simply short-lived leukemic progenitors.
Frequency of LICs in each fraction
The frequency of LICs in specific fractions was determined by limiting dilution analysis of 4 sorted samples (Table 6). The LIC frequency was not significantly different between the CD34−CD38− and CD34−CD38+ fractions from 2 subtype A samples (P > .3 for each). The frequency of LICs was significantly lower (P < .05 for each) in the CD34−CD38+ fraction than the other 3 fractions for one subtype C AML (sample 17). There was no significant difference between the CD34−CD38− fraction and the CD34+CD38− or the CD34+CD38+ fraction for this sample. When the number of LICs per fraction per 106 mononuclear cells was calculated, the majority of LICs were found to fall within the CD34− fractions as these are much larger than the CD34+ fractions (Table 6). The number of LICs per 106 mononuclear cells was approximately 7-fold higher for the CD34+ fraction compared with the CD34− fraction from an additional subtype C sample with high CD34 expression (sample 25).
Table 6.
LIC frequency in sorted fractions of AML
| AML | Fraction | Dose |
LIC frequency (95% confidence interval) | LICs per 106 MNCs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 102 cells | 103 cells | 104 cells | 2 × 104 cells | 5 × 104 cells | 105 cells | 5 × 105 cells | 106 cells | 3 × 106 cells | ||||
| 3 | CD34−CD38− | 2/4 | 3/4 | 3/3 | 3/3 | 3/3 | 1 in 458 (153-1369) | 432 | ||||
| 3 | CD34−CD38+ | 0/4 | 3/4 | 4/4 | 3/3 | 3/3 | 1 in 873 (275-2768) | 918 | ||||
| 9 | CD34−CD38− | 0/3 | 4/4 | 4/4 | 1 in 245 454 (85 866-701 648) | 2.2 | ||||||
| 9 | CD34−CD38+ | 0/4 | 2/5 | 2/2 | 3/3 | 1 in 231 326 (83 726-639 131) | 1.8 | |||||
| 17 | CD34−CD38− | 0/1 | 1/3 | 4/4 | 4/4 | 4/4 | 4/4 | 1 in 2164 (538-8698) | 228 | |||
| 17 | CD34−CD38+ | 1/3 | 3/4 | 4/4 | 3/3 | 1 in 58 407 (19 219-177 497) | 8.5 | |||||
| 17 | CD34+CD38− | 2/3 | 6/8 | 1 in 540 (236-1235) | 11 | |||||||
| 17 | CD34+CD38+ | 3/5 | 2/2 | 1 in 1091 (339-3506) | 2.3 | |||||||
| 25 | CD34− | 0/1 | 0/2 | 0/3 | 2/2 | 1 in 3 327 588 (885 486-12 504 816) | 0.1 | |||||
| 25 | CD34+ | 0/3 | 1/2 | 2/3 | 2/2 | 1 in 878 831 (321 224-2 404 374) | 0.7 | |||||
NOD/SCID/IL2rγ−/− mice were used as hosts for samples 3 and 9. NOD/SCID/β2m−/− mice were used as hosts for samples 17 and 25. Mice were sacrificed at 10 weeks for sample 25, 12 weeks for samples 3 and 17, and 15 weeks for sample 9.
Data are reported as number of mice with AML/number of mice receiving transplant.
Normal CD34−CD38+ cells do not contain repopulating cells
As all samples had LICs within the CD34−CD38+fraction (one sample had LICs restricted to this fraction), we tested whether this population of normal hematopoietic tissue has any repopulating capacity in our more immunodeficient mouse model (IVIG-treated NOD/SCID/IL2rγ−/− mice); 106 CD3−CD34−CD38+ cells from cord blood were transplanted but failed to produce a graft at 20 days (3 mice), 9 weeks (4 mice), and 19 weeks (1 mouse). Similarly, 4.3 × 104 lineage negative CD34−CD38+ cord blood cells failed to produce a graft at 9 and 19 weeks, whereas 1.4-4.3 × 104 lineage negative CD34+CD38− cells gave rise to multilineage engraftment at both these time points (data not shown).
The immunophenotype of LICs can change after xenotransplant
To assess whether the transplanted cells recapitulate the original leukemia, we examined the expression of myelo-monocytic markers on human CD45+ cells present in the bone marrow of mice successfully transplanted with subfractions of AML. The leukemia cells expressed mature myelo-monocytic markers (CD14, CD15, CD36) consistent with the French American British classification (FAB) subtype of the original sample (supplemental Table 3). Myelo-monocytic and monocytic/blastic subtypes (M4 and M5) expressed mature monocytic markers, whereas myeloblastic subtypes (M1 and M2) expressed CD33 but only a small minority of cells expressed the mature myelo-monocytic markers (supplemental Figure 3).
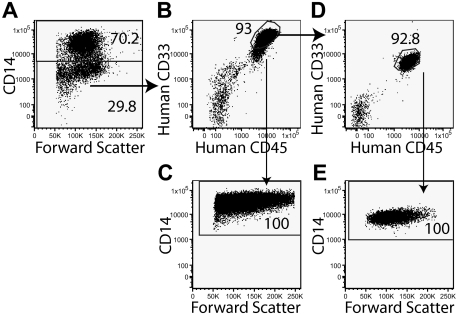
However, we noted some changes in the phenotype of engrafted AML cells. Only one of 13 samples (sample 19) maintained expression of CD34. The majority of engrafted leukemia cells had a CD34−CD38+phenotype (Figure 2). By contrast, a CD34+ population was seen in the normal graft derived from the CD34+CD38− fraction of sample 15 (Figure 2Biv). Given that the CD34+ fraction from some subtype C samples initiated leukemia in primary and secondary recipients and yet engrafted cells did not express CD34, the data suggest that the CD34+ LICs had lost CD34 expression in the mice. Similarly, LICs from sample 7 were shown to lack CD14 expression in the primary sample but become CD14+ within the mice (Figure 3).
Figure 3.
The phenotype of LICs can change in the mice. The expression of CD14 is seen in the primary AML sample from sample 7(A). The sample was sorted, and 250 000 CD14+ cells were transplanted into 3 mice, but no graft was detected at 9 weeks. By contrast 250 000 CD14− cells gave rise to a graft at 9 weeks in 2 of 2 mice transplanted (B), indicating that the LICs were CD14−. All the human CD45+ cells derived from the CD14− fraction expressed CD14 (C). These CD14+ cells were capable of initiating a graft in secondary recipients (D), indicating that the LICs were now CD14. The human CD45+ cells in the secondary recipients were CD14+ (E).
NPM wild-type AMLs with low CD34 expression have CD34− LICs
Although the expression of CD34 is significantly higher in NPM wild-type AML, some NPM wild-type samples have low expression of CD34. We sorted 2 such wild-type NPM samples (termed WT1 and WT2) and transplanted the cells into immunodeficient mice. The LICs were restricted to the CD34− fraction (Tables 3 and 4). The immunophenotype of the graft from WT1 resembled that derived from the subtype A NPM-mutated samples, whereas WT2 comprised mostly CD34−CD38− cells.
Discussion
LICs were found within the CD34− fraction of all 15 AMLs (NPM-mutated) that were studied in vivo. Whereas all 6 subtype A samples had LICs restricted to the CD34− fraction, all subtype C samples had both CD34+ and CD34− LICs. These LICs were capable of initiating leukemia in secondary recipients and so are not simply short-lived leukemic progenitors.
The apparent discrepancy between this and earlier studies is due to our focus on NPM-mutated AML and the improved tools we used (more immunodeficient mice, measures to abrogate the inhibitory effects of anti-CD38 antibody, and improved graft identification). Conventional NOD/SCID mice can only act as a host for some AMLs, notably those with a worse prognosis.19 Consequently, earlier studies may have been biased away from samples with relatively favorable features such as NPM mutation5 and low CD34 expression24 (< 50% of NPM mutant AMLs engraft NOD/SCID mice19). By contrast, the newer strains can act as a host for more than 80% of NPM-mutated AML. There may have been a further bias against subtype A samples relative to subtype C samples, as the subtype A samples have a lower incidence of FLT3 ITD, a poor risk mutation.
Using IVIG to abrogate the effects of anti-CD38 antibody, we noted that LICs were found in the CD34+CD38− fraction and either of the 2 CD38+ fractions from 3 of 6 subtype C samples. It is conceivable that the CD38+ LICs (CD34+CD38+ and/or CD34−CD38+) from some subtype C samples may have been eliminated by the anti-CD38 antibody3 in earlier studies, leading to the impression that only CD34+CD38− LICs exist.2 Consistent with this explanation, 2 studies in which anti-CD38 antibody was not used showed LICs in both CD34− and CD34+ fractions from some samples.25,26
Lastly, the methodology used in some earlier studies could not always determine whether grafts were leukemic or normal (from normal CD34+ cells within the leukemia sample19,27,28). Most early studies did not use immunophenotyping to identify the lineage of engrafted human CD45+ cells and so could not readily discriminate between normal multilineage engraftment and unilineage AML. Cytogenetic and molecular techniques would not have added much, as most NPM-mutated AMLs have a normal karyotype (and NPM mutation was not identified until later4). Therefore, some grafts from the CD34+ fractions of AML may have been normal but misclassified as leukemic.
More than one fraction initiated AML from 10 samples (Table 4). This probably reflects changes in expression of CD34 and CD38 on LICs under different conditions or at different times. Mouse hematopoietic stem cells (HSCs) show variation in the expression of these markers after exposure to chemotherapy or after mobilization by granulocyte colony-stimulating factor.29 Similarly CD34 is expressed on HSCs from young mice but is lost as mice age.30 The CD34+ fraction from sample 17 was able to initiate AML in primary and secondary recipients, yet the engrafted cells lacked CD34. Thus the LICs were initially CD34+ but became CD34− within the mouse bone marrow environment. The mice appear to push the LIC phenotype toward a more differentiated one with increased CD38, reduced CD34, and in the case of monocytic leukemias increased CD14. Similar changes may occur in man depending on microenvironmental conditions.
Nevertheless, the mouse bone marrow environment does not reduce CD34 expression per se, as grafts derived from normal repopulating cells and some AMLs have CD34+ populations (with repopulating ability).1,2 Therefore, the loss of the CD34+ from leukemic cells within the mice suggests that the expression of CD34 is not a stable feature of the LICs from most subtype B and C samples (in contrast to LICs from some other AMLs).
An alternative explanation why multiple fractions initiate leukemia is that there may be several different LICs with distinct properties within one leukemia sample. LICs with different phenotypes may have evolved from the original LICs through acquisition of additional hits. The evolution of new generations of LICs has been observed using an in vivo model of acute leukemia derived from MLL-transformed cord blood cells.31
The AMLs with exclusively CD34− LICs may have arisen from normal CD34+ stem or progenitor cells but have lost expression of CD34 through the transforming events or as a result of the microenvironment.31,32 Alternatively, these AMLs may derive from CD34− long-term repopulating cells that have been described in human bone marrow33 and cord blood34 or from CD34− myeloid cells that have gained self-renewal potential. However, it is not possible from the data we have presented to clarify this issue.
In conclusion, we have demonstrated that the phenotype of LICs from NPM-mutated AMLs is different to that previously reported for unselected AMLs. In some the LICs were exclusively CD34−, whereas other samples had both CD34− and CD34+ LICs. The study reinforces the idea that there is greater heterogeneity in the phenotype of LICs than previous studies indicated. LICs are not found in the CD34+CD38− fraction in approximately half of NPM-mutated AMLs, and this phenotype cannot be blindly relied upon to prospectively identify LICs. Moreover, we have demonstrated that LICs can change their phenotype, and this may well explain the observation that LICs are found in multiple fractions from some AMLs. This property will mean that LICs will be harder to completely eliminate using targeted therapies such as monoclonal antibodies.
Acknowledgments
We are indebted to patients who gave samples. We thank Dr. Michael Jenner for providing diagnostic information.
D.C.T. is supported by a Medical Research Council Clinician Scientist Fellowship. This work was supported by Cancer Research UK and a European Commission Grant (LSHC-CT-206-037632; D.B.) and by funding from National Cancer Institute (P01 CA95426; J.G.G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.C.T. designed and performed research, analyzed and interpreted data, performed statistical analysis and wrote the manuscript; J.V. performed research, and analyzed and interpreted data; F.M.-M., E.G., K.S. and T.L. performed research; D.L. provided vital data; H.O. and J.C. provided vital materials; S.G.A. contributed vital materials; T.A.L. was responsible for the Tissue Bank and clinical database; J.G.G. interpreted data and wrote the manuscript; and D.B. designed research and analyzed, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominique Bonnet, Haematopoietic Stem Cell Laboratory, CRUK London Research Institute, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom; e-mail: dominique.bonnet@cancer.org.uk; or David Taussig, CRUK Medical Oncology Unit, Room 310, John Vane Building, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: d.taussig@qmul.ac.uk.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 3.Taussig DC, Miraki-Moud F, Anjos-Afonso F, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112(3):568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 4.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 5.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 6.Falini B, Martelli MP, Mecucci C, et al. Cytoplasmic mutated nucleophosmin is stable in primary leukemic cells and in a xenotransplant model of NPMc+ acute myeloid leukemia in SCID mice. Haematologica. 2008;93(5):775–779. doi: 10.3324/haematol.12225. [DOI] [PubMed] [Google Scholar]
- 7.Chou WC, Tang JL, Lin LI, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res. 2006;66(6):3310–3316. doi: 10.1158/0008-5472.CAN-05-4316. [DOI] [PubMed] [Google Scholar]
- 8.Schneider F, Hoster E, Unterhalt M, et al. NPM1 but not FLT3-ITD mutations predict early blast cell clearance and CR rate in patients with normal karyotype AML (NK-AML) or high-risk myelodysplastic syndrome (MDS). Blood. 2009;113(21):5250–5253. doi: 10.1182/blood-2008-09-172668. [DOI] [PubMed] [Google Scholar]
- 9.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006;107(10):4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Mecucci C, Saglio G, et al. NPM1 mutations and cytoplasmic nucleophosmin are mutually exclusive of recurrent genetic abnormalities: a comparative analysis of 2562 patients with acute myeloid leukemia. Haematologica. 2008;93(3):439–442. doi: 10.3324/haematol.12153. [DOI] [PubMed] [Google Scholar]
- 11.Alcalay M, Tiacci E, Bergomas R, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005;106(3):899–902. doi: 10.1182/blood-2005-02-0560. [DOI] [PubMed] [Google Scholar]
- 12.Garzon R, Garofalo M, Martelli MP, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci U S A. 2008;105(10):3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haferlach C, Mecucci C, Schnittger S, et al. AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biological, pathological, immunophenotypic, and prognostic features. Blood. 2009;114(14):3024–3032. doi: 10.1182/blood-2009-01-197871. [DOI] [PubMed] [Google Scholar]
- 14.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 15.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–3080. [PubMed] [Google Scholar]
- 16.Virappane P, Gale R, Hills R, et al. Mutation of the Wilms' tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: the United Kingdom Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2008;26(33):5429–5435. doi: 10.1200/JCO.2008.16.0333. [DOI] [PubMed] [Google Scholar]
- 17.Schraml E, Daxberger H, Watzinger F, Lion T. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Vienna experience. Leukemia. 2003;17(1):224–227. doi: 10.1038/sj.leu.2402756. [DOI] [PubMed] [Google Scholar]
- 18.Chou WC, Tang JL, Wu SJ, et al. Clinical implications of minimal residual disease monitoring by quantitative polymerase chain reaction in acute myeloid leukemia patients bearing nucleophosmin (NPM1) mutations. Leukemia. 2007;21(5):998–1004. doi: 10.1038/sj.leu.2404637. [DOI] [PubMed] [Google Scholar]
- 19.Pearce DJ, Taussig D, Zibara K, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107(3):1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taussig DC, Pearce DJ, Simpson C, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106(13):4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yahata T, Ando K, Sato T, et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101(8):2905–2913. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1-2):70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Martelli MP, Manes N, Pettirossi V, et al. Absence of nucleophosmin leukaemic mutants in B and T cells from AML with NPM1 mutations: implications for the cell of origin of NPMc+ AML. Leukemia. 2008;22(1):195–198. doi: 10.1038/sj.leu.2404857. [DOI] [PubMed] [Google Scholar]
- 24.Repp R, Schaekel U, Helm G, et al. Immunophenotyping is an independent factor for risk stratification in AML. Cytometry B Clin Cytom. 2003;53(1):11–19. doi: 10.1002/cyto.b.10030. [DOI] [PubMed] [Google Scholar]
- 25.Terpstra W, Prins A, Ploemacher RE, et al. Long-term leukemia-initiating capacity of a CD34-subpopulation of acute myeloid leukemia. Blood. 1996;87(6):2187–2194. [PubMed] [Google Scholar]
- 26.Blair A, Hogge DE, Sutherland HJ. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(−)/HLA-DR. Blood. 1998;92(11):4325–4335. [PubMed] [Google Scholar]
- 27.Ailles LE, Gerhard B, Hogge DE. Detection and characterization of primitive malignant and normal progenitors in patients with acute myelogenous leukemia using long-term coculture with supportive feeder layers and cytokines. Blood. 1997;90(7):2555–2564. [PubMed] [Google Scholar]
- 28.Feuring-Buske M, Hogge DE. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34(+)CD38(−) progenitor cells from patients with acute myeloid leukemia. Blood. 2001;97(12):3882–3889. doi: 10.1182/blood.v97.12.3882. [DOI] [PubMed] [Google Scholar]
- 29.Tajima F, Deguchi T, Laver JH, Zeng H, Ogawa M. Reciprocal expression of CD38 and CD34 by adult murine hematopoietic stem cells. Blood. 2001;97(9):2618–2624. doi: 10.1182/blood.v97.9.2618. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka S, Ebihara Y, Xu M, et al. CD34 expression on long-term repopulating hematopoietic stem cells changes during developmental stages. Blood. 2001;97(2):419–425. doi: 10.1182/blood.v97.2.419. [DOI] [PubMed] [Google Scholar]
- 31.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316(5824):600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 32.Wei J, Wunderlich M, Fox C, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13(6):483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26(4):353–360. [PubMed] [Google Scholar]
- 34.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4(9):1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]