Abstract
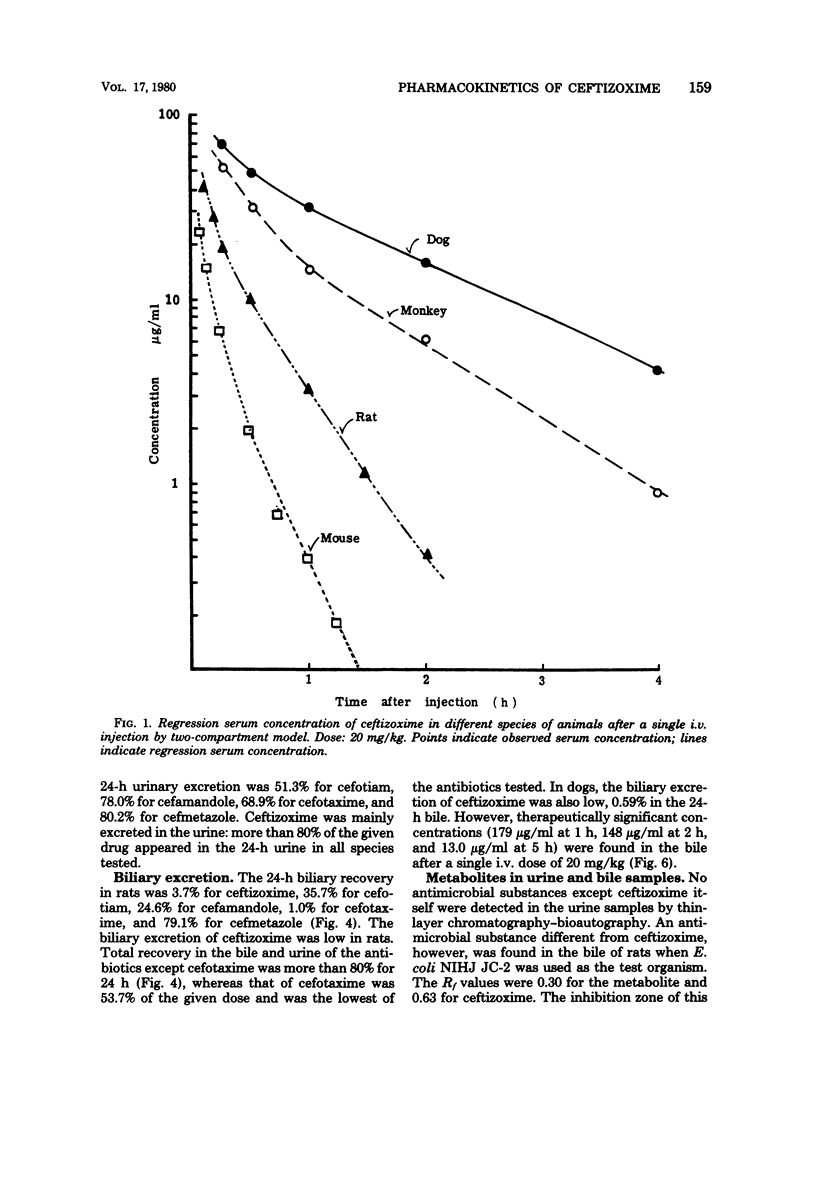
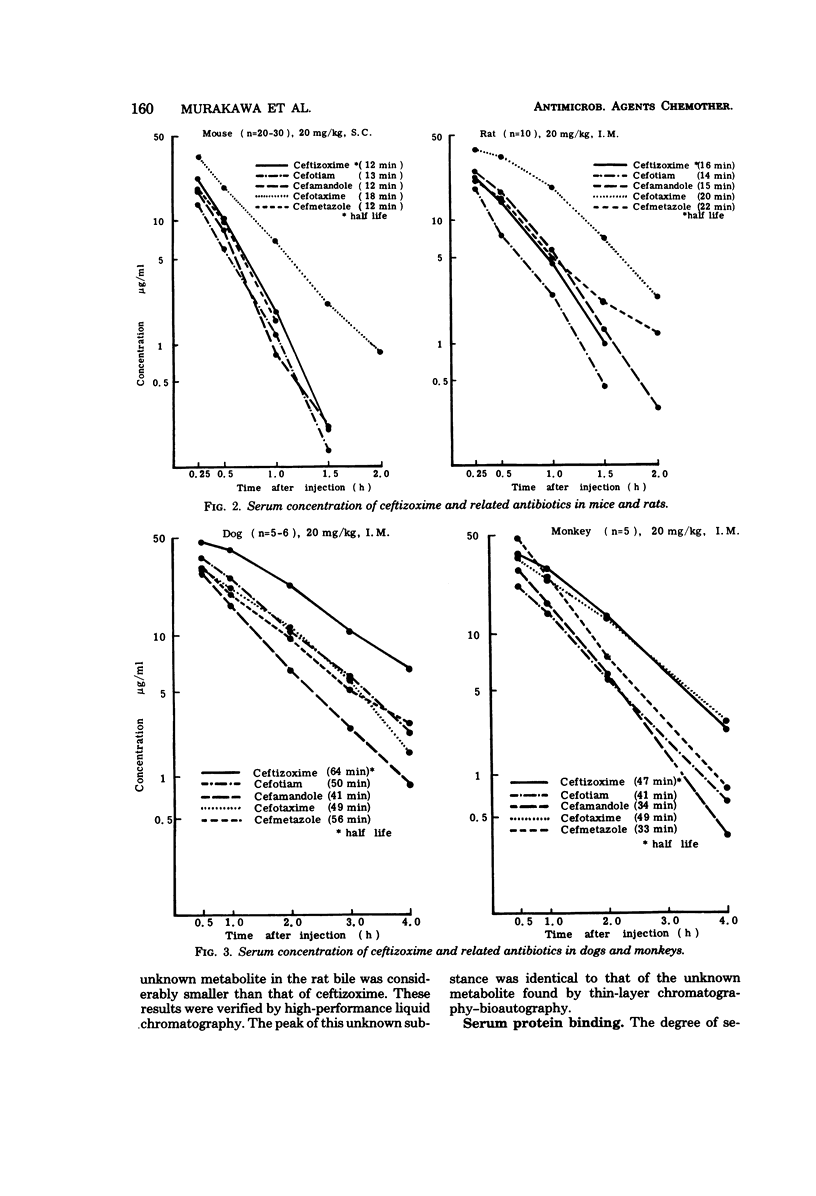
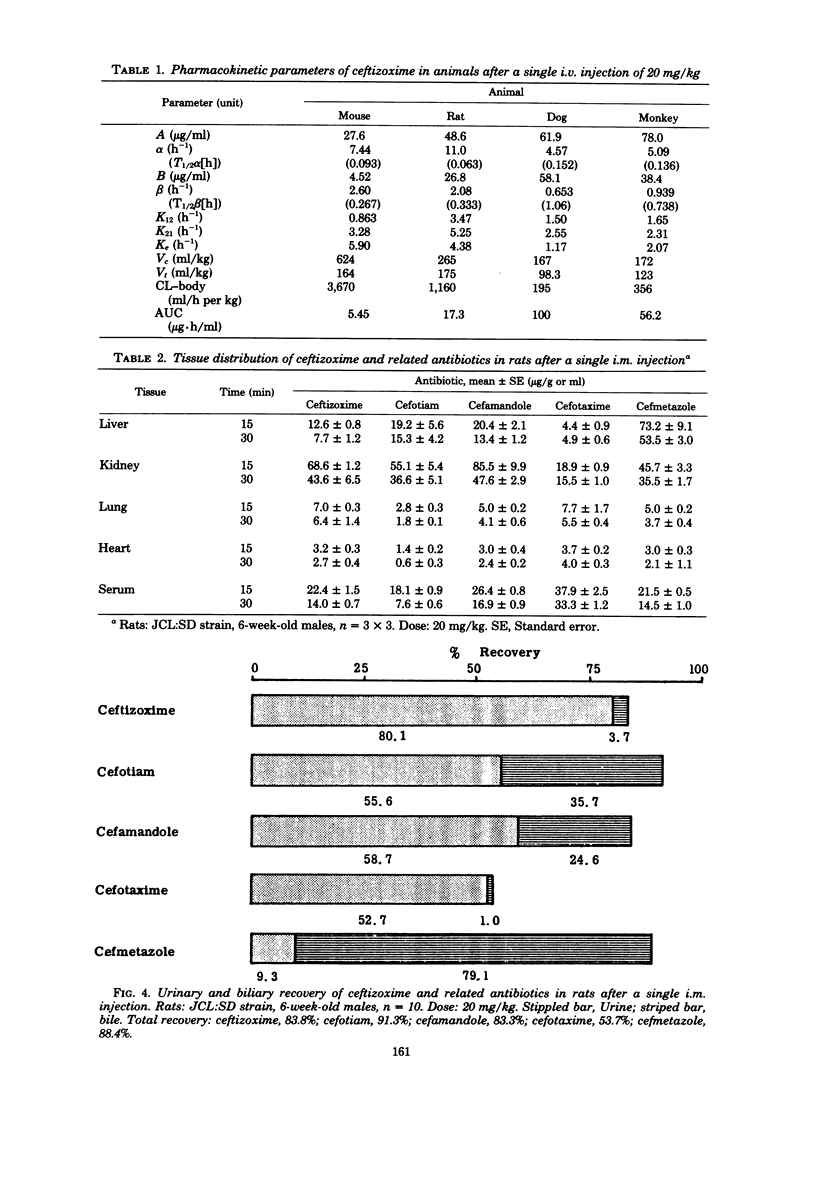
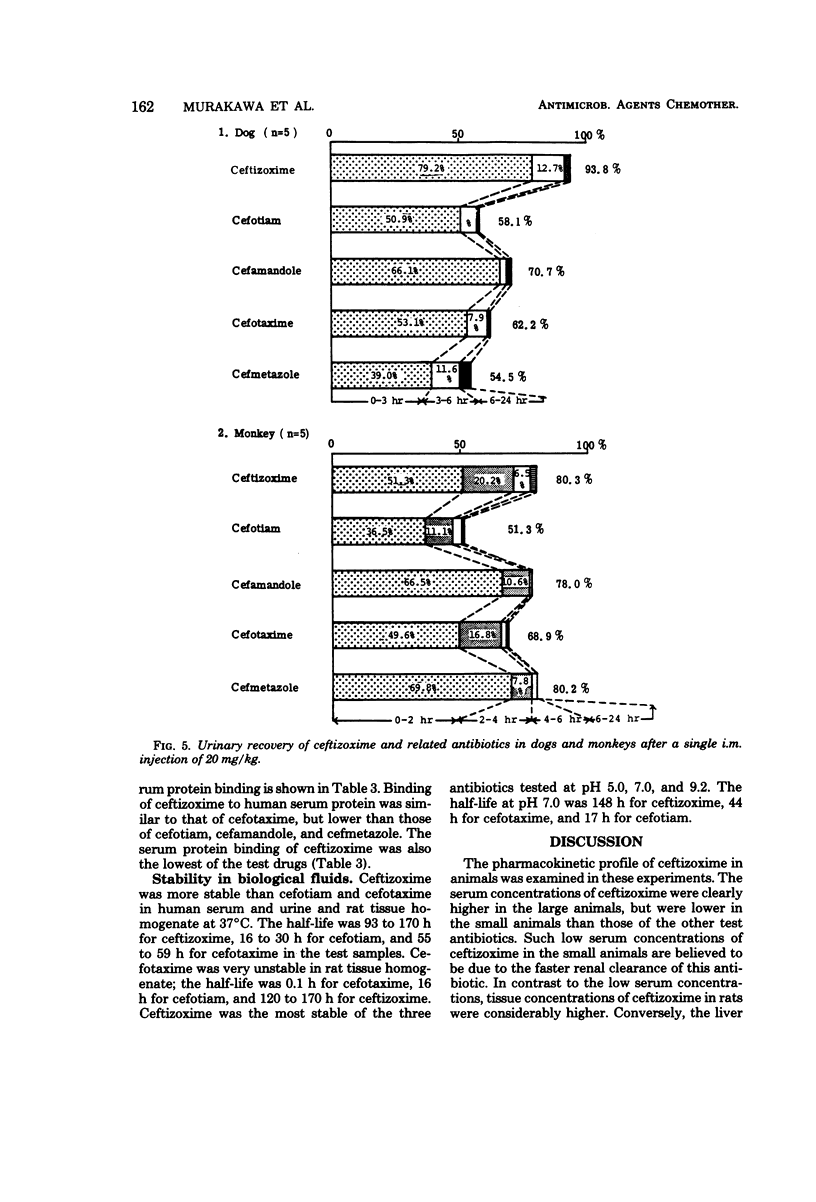
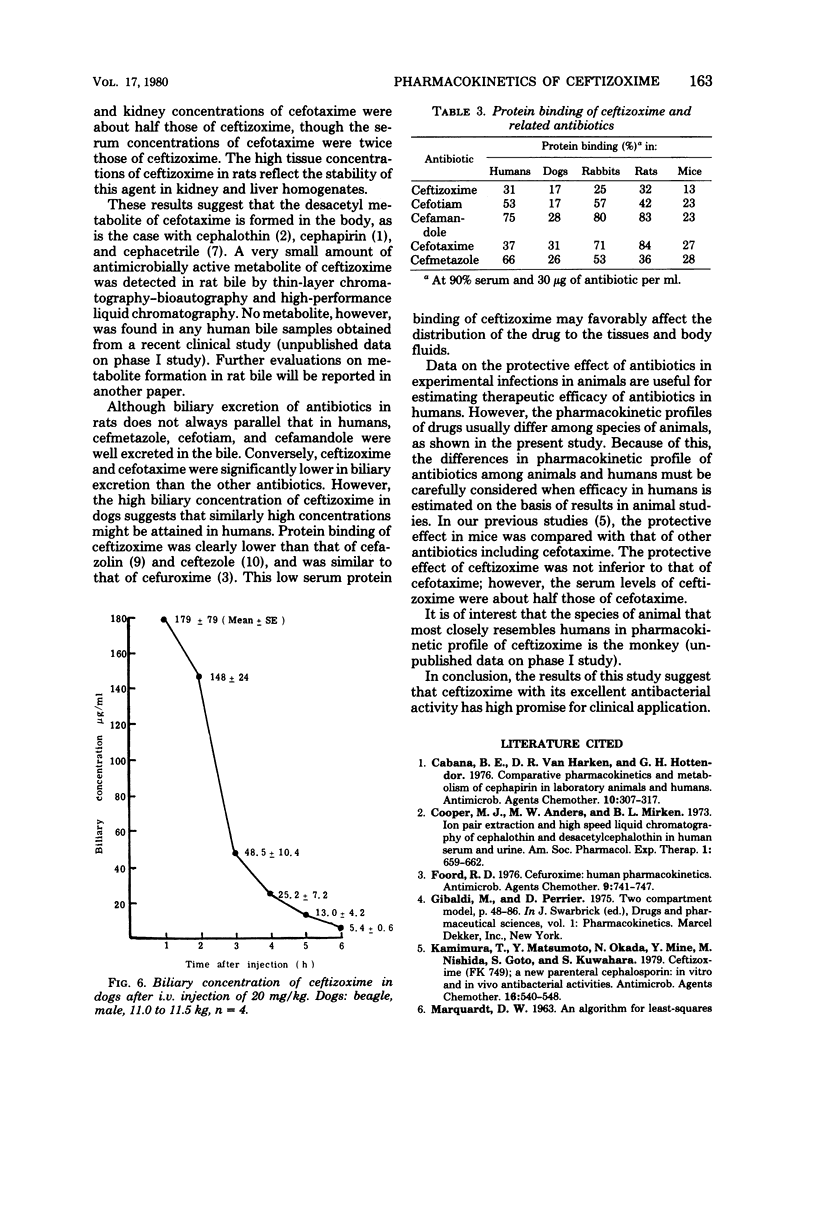
The pharmacokinetic profile of ceftizoxime was studied in mice, rats, dogs, and monkeys given the drug in a single parenteral dose. The serum data after an intravenous injection were analyzed by the two-compartment open model. Cefotiam, cefmetazole, cefotaxime, and cefamandole were used as reference drugs. High concentrations of ceftizoxime were attained in the sera of all test animals and in the tissues of rats after parenteral dosing. The serum concentrations of ceftizoxime were higher than those of the other antibiotics in large animals (dogs and monkeys), but were lower in small animals (mice and rats). About 80% of ceftizoxime was excreted unchanged in the 24-h urine of all species tested. The biliary excretion of ceftizoxime was low: 3.7% in rats and 0.59% in dogs. However, therapeutically significant concentrations of ceftizoxime were found in the bile of dogs. Ceftizoxime was stable in biological fluids such as serum, urine, and tissue homogenates, but cefotaxime was unstable in rat tissue homogenates. Binding of ceftizoxime to serum protein in all species was the lowest of all the antibiotics: 31% for humans, 17% for dogs, and 32% for rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabana B. E., van Harken D. R., Hottendorf G. H. Comparative pharmacokinetics and metabolism of cephapirin in laboratory animals and humans. Antimicrob Agents Chemother. 1976 Aug;10(2):307–317. doi: 10.1128/aac.10.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. J., Anders M. W., Mirkin B. L. Ion-pair extraction and high-speed liquid chromatography of cephalothin and deacetylcephalothin in human serum and urine. Drug Metab Dispos. 1973 Sep-Oct;1(5):659–662. [PubMed] [Google Scholar]
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Matsumoto Y., Okada N., Mine Y., Nishida M., Goto S., Kuwahara S. Ceftizoxime (FK 749), a new parenteral cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1979 Nov;16(5):540–548. doi: 10.1128/aac.16.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T., Waki Y., Nishida M., Fuji R., Konno M. Chromatographic assay of mixed penicillins, ampicillin and cloxacillin in body fluids. J Antibiot (Tokyo) 1970 May;23(5):250–251. doi: 10.7164/antibiotics.23.250. [DOI] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y. Cefazolin, a new semisynthetic cephalosporin antibiotic. II. In vitro and in vivo antimicrobial activity. J Antibiot (Tokyo) 1970 Mar;23(3):137–148. doi: 10.7164/antibiotics.23.137. [DOI] [PubMed] [Google Scholar]
- Nishida M., Murakawa T., Kamimura T., Okada N., Sakamoto H., Fukada S., Nakamoto S., Yokota Y., Miki K. In vitro and in vivo evaluation of ceftezole, a new cephalosporin derivative. Antimicrob Agents Chemother. 1976 Jul;10(1):1–13. doi: 10.1128/aac.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


