Abstract
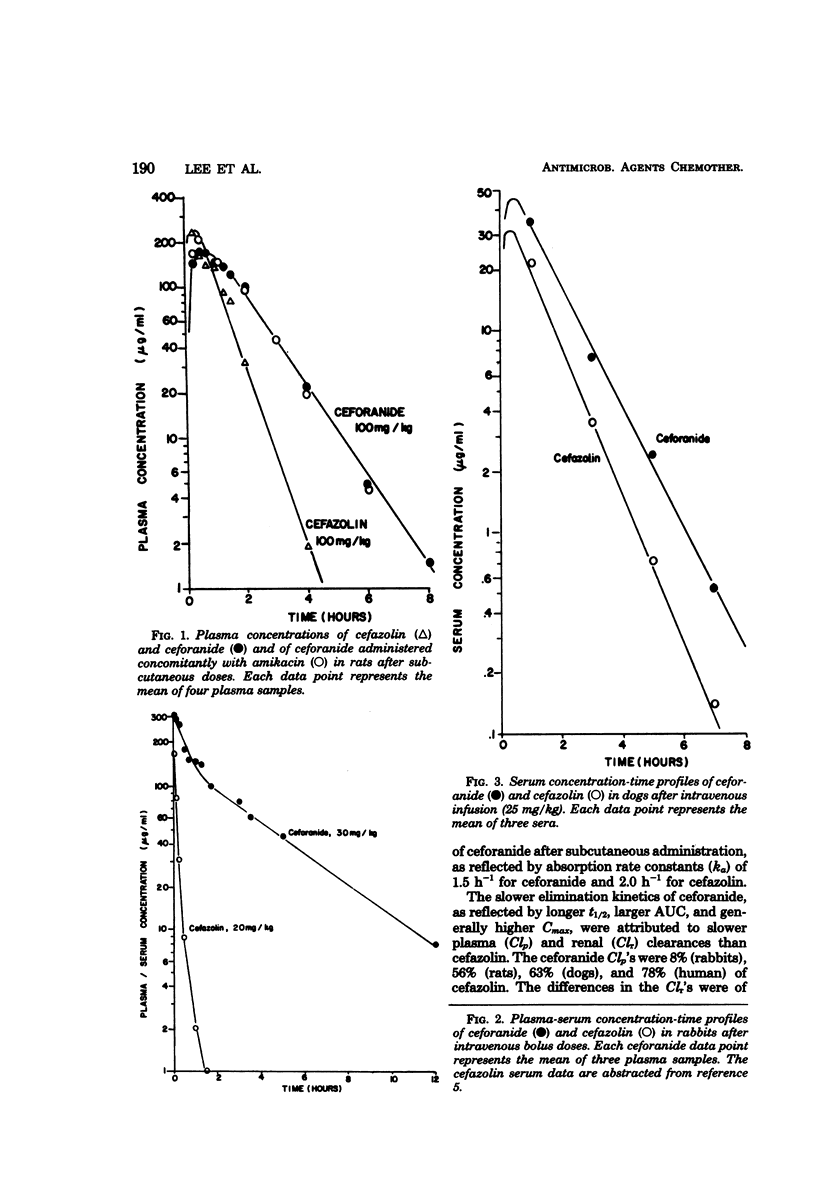
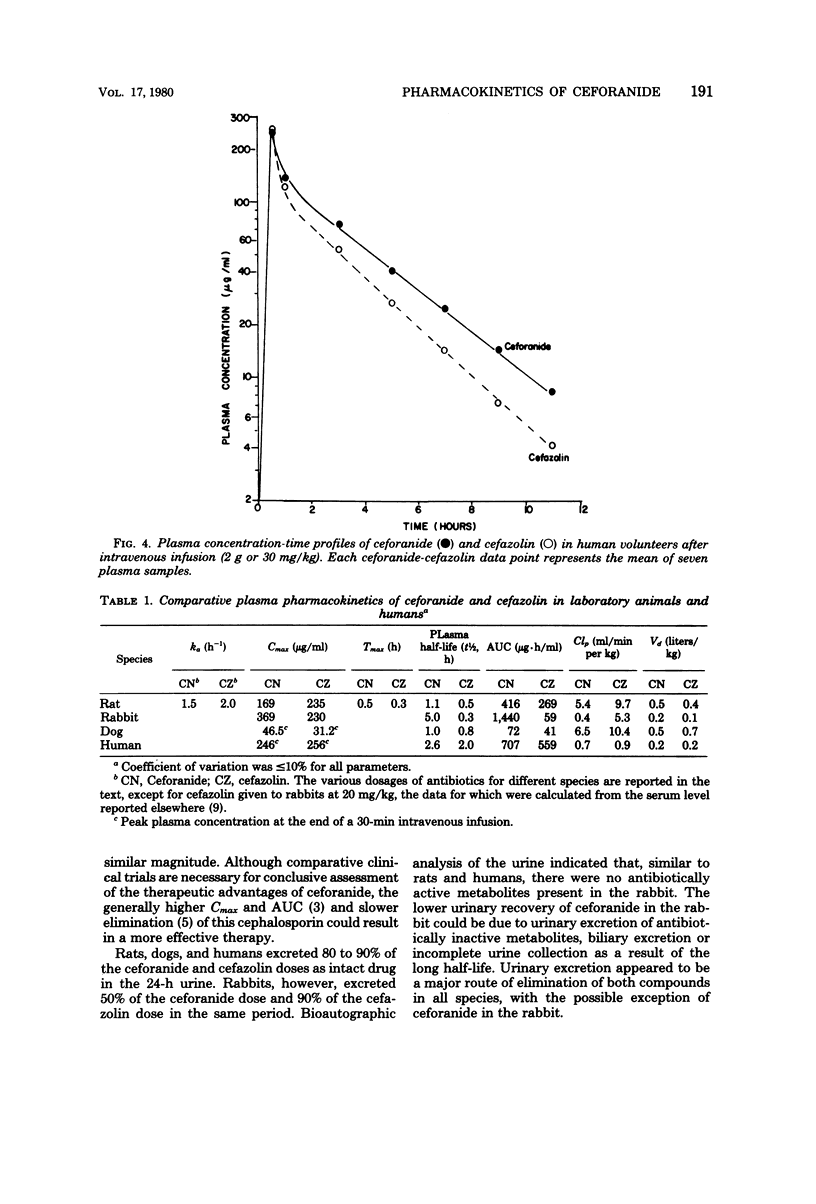
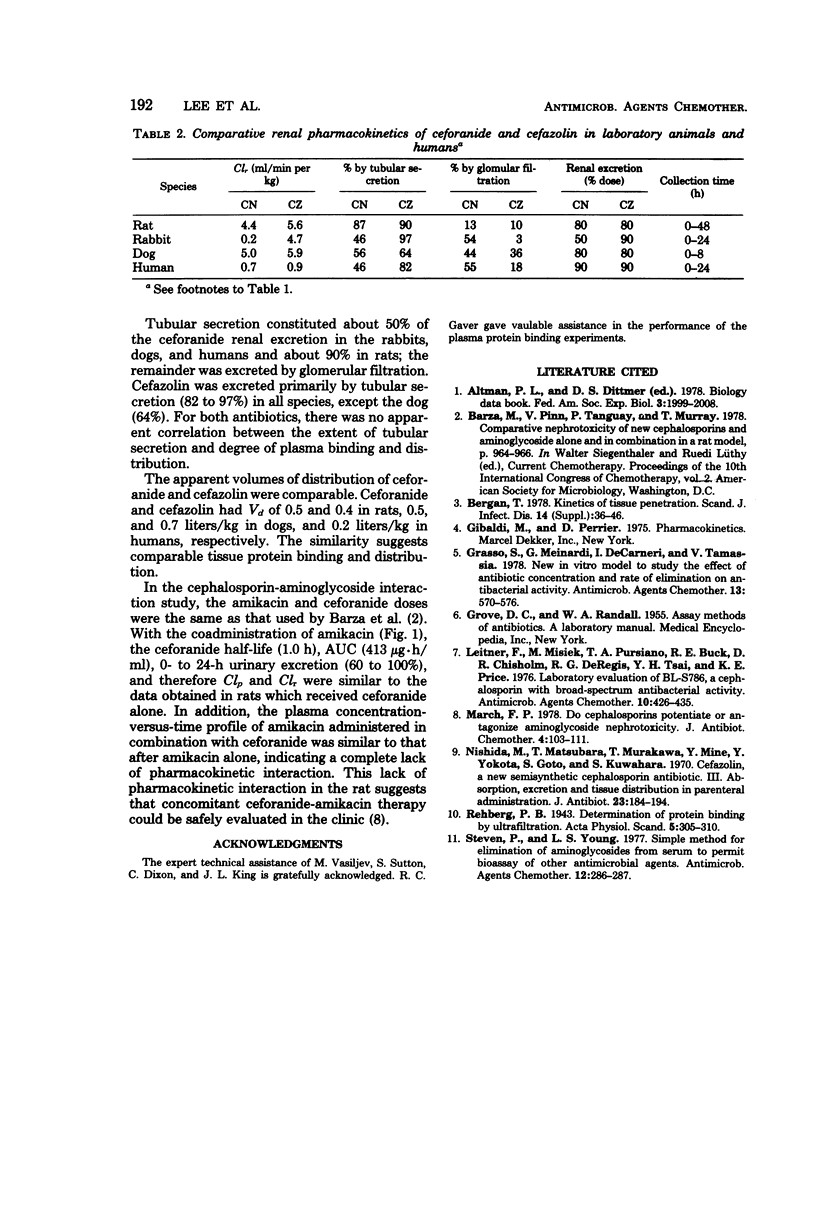
Ceforanide (BL-S786R) is a new, broad-spectrum, parenteral cephalosporin. Pharmacokinetic properties were determined in rats (100 mg/kg), rabbits (30 mg/kg), dogs (25 mg/kg), and humans (2 g or 30 mg/kg) and compared with equivalent single doses of cefazolin. Plasma half-lives for ceforanide and cefazolin were 1.1 and 0.5 h in the rat, 5 and 0.3 h in the rabbit, 1 and 0.8 h in the dog, and 2.6 and 2 h in humans, respectively. The slower elimination of ceforanide, as reflected by longer plasma half-life, larger area under the curve, and peak plasma concentrations, was due to slower body and renal clearances. The apparent volumes of distribution of ceforanide and cefazolin were comparable. Rats, dogs, and humans excreted 80 to 100% of the ceforanide dose in the 0- to 24-h urine; rabbits excreted only 50%. Tubular secretion constituted 50% of ceforanide renal excretion in rabbits, dogs, and humans and 90% in rats; the remainder was excreted by glomerular filtration. There was no apparent correlation between the extent of tubular secretion and degree of plasma protein binding in different species. There was no significant pharmacokinetic interaction between ceforanide and amikacin in the rat. The slower elimination kinetics of ceforanide are indicative of the potential for a longer dosing interval and more effective antibiotic therapy as compared with available cephalosporins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T. Kinetics of tissue penetration. Are high plasma peak concentrations or sustained levels preferable for effective antibiotic therapy? Scand J Infect Dis Suppl. 1978;(14):36–46. [PubMed] [Google Scholar]
- Grasso S., Meinardi G., de Carneri I., Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978 Apr;13(4):570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Misiek M., Pursiano T. A., Buck R. E., Chisholm D. R., DeRegis R. G., Tsai Y. H., Price K. E. Laboratory evaluation of BL-S786, a cephalosporin with broad-spectrum antibacterial activity. Antimicrob Agents Chemother. 1976 Sep;10(3):426–435. doi: 10.1128/aac.10.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Goto S., Kuwahara S. Cefazolin, a new semisynthetic cephalosporin antibiotic. 3. Absorption, excretion and tissue distribution in parenteral administration. J Antibiot (Tokyo) 1970 Apr;23(4):184–194. [PubMed] [Google Scholar]
- Stevens P., Young L. S. Simple method for elimination of aminoglycosides from serum to permit bioassay of other antimicrobial agents. Antimicrob Agents Chemother. 1977 Aug;12(2):286–287. doi: 10.1128/aac.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]


