Abstract
Purpose
Aplidin (plitidespin) is a novel cyclic depsipeptide, currently in Phase II clinical trials for solid and hematologic malignancies. We examined the effects of oxygen on the cytotoxicity of Aplidin and the interactions between Aplidin and radiation. These factors will be important if Aplidin is used clinically in combination with radiotherapy.
Materials
Exponentially-growing EMT6 mouse mammary tumour cells in monolayer cultures were treated with Aplidin and 250 kV X-rays.
Results
The cytotoxicity of Aplidin was not altered either by incubation in moderate hypoxia before and during a 24 h drug treatment or by incubation in severe hypoxia before and during a 2 h drug treatment. Treatment with Aplidin plus radiation produced cytotoxicities compatible with additive or supraadditive cytotoxicities. Cells treated with 1 μM Aplidin for 24 h then killed by 100 Gy of radiation were toxic to untreated cells co-cultured with them.
Conclusions
The cytotoxicity of Aplidin is independent of the oxygenation during treatment. Aplidin, or an active metabolite of Aplidin, is retained in the cells and later released as the radiation-sterilised cells die, producing a Bystander effect that kills neighbouring cells. This Bystander effect could affect the outcome of therapeutic regimens combining Aplidin and radiation.
Keywords: Aplidin, radiation, oxygenation, Bystander effects
Introduction
Aplidin (plitidespin) is a novel cyclic depsipeptide originally isolated from the marine tunicate Aplidium albicans (Rinehart 2000, García-Fernández et al. 2002). Aplidin has been shown to have anti-tumour activity in a variety of human and murine tumour cell lines, murine tumours and human tumour xenografts (Rinehart 2000, García-Fernández et al. 2002, Mitsiades et al. 2008); its mechanism of action has not been fully elucidated (Biscardi et al. 2005, Gajate and Mollinedo 2005, González-Santiago et al. 2006, 2007, Straight et al. 2006, Suárez et al. 2006, Humeniuk et al. 2007, Moneo et al. 2007, Muñoz-Alonso et al. 2008). Aplidin is currently in Phase II clinical trials in a variety of solid and hematologic malignancies and has been shown to have clinical anti-tumour activity in advanced melanoma, metastatic clear cell renal carcinoma, peripheral T-cell lymphoma (PTCL) and multiple myeloma (Rinehart 2000, García-Fernández et al. 2002, Mitsiades et al. 2008). The drug has recently been granted orphan drug designation by the European Medicines Agency and the US Food and Drug Administration for the treatment of acute lymphoblastic leukemia and multiple myeloma. The main side-effects of Aplidin in clinical trials have been myalgia and muscle weakness as well as transient and reversible transaminase elevations; interestingly, marrow toxicity is very mild and has not been dose limiting (Rinehart 2000, García-Fernández et al. 2002, Mitsiades et al. 2008).
This paper presents our preliminary cell culture studies examining two aspects of the effects of Aplidin that have not been studied previously: Its interactions with radiation and the dependence of its cytotoxic effects on oxygen. These questions will be increasingly important as Aplidin is used more widely in the treatment of solid tumours.
Radiation therapy is widely used in the curative and palliative treatment of solid malignancies. At present, about 65% of all cancer patients receive radiotherapy at some point during their treatment, alone or in combination with surgery and/or chemotherapy. Increasingly, combined modality regimens are moving toward the use of concomitant administration of agents, rather than separated, sequential therapies. As Aplidin enters broader use in the clinic, it is likely that this drug will be used in patients who also receive radiation therapy. This makes it important to examine the effects of regimens combining Aplidin with radiation, to define any interactions between the two agents.
Hypoxia is a common feature of solid tumours in animals and in human patients (Arbeit et al. 2006, Dewhirst 2009, Rockwell et al. 2009). Tumours develop regions of severe hypoxia (with oxygen tensions below 1 Torr) very early in their development. Primary and metastatic tumours with diameters less than 1 mm have already developed severe hypoxia. As solid tumours grow, they must elicit the development of vascular beds to support the malignant cells through angiogenesis, neovascularisation, and co-option of vessels from normal tissues. However, the vascular bed that develops during this process lacks the organisation and regulation found in the vasculature that supports healthy normal tissues. The vessels are tortuous and irregular; blind ends, shunting and other defects are common. The vessels also lack the musculature needed to precisely regulate blood flow and frequently have microscopic leaks or even macroscopic holes that permit plasma or blood to escape into the surrounding tissue. In addition, the growing tumour may invade or compress the blood and lymphatic vessels, further compromising their functionality. As a result, perfusion within solid tumours is abnormal and inadequate, and tumours contain regions where temporary interruptions in blood flow through individual vessels or persistent deficiencies in perfusion lead to transient and chronic hypoxia. Cells in these regions are resistant to radiation and to many anti-cancer drugs. Drugs that attack these resistant cells can be valuable in combination with radiotherapy or with the standard anticancer drugs that are preferentially toxic to the aerobic cells. Because Aplidin has been shown to alter the function of VEGF/VEGFR (vascular endothelial growth factor and its receptor), alter oxidative metabolism, deplete reduced glutathione, and alter mitochondrial function (Biscardi et al. 2005, González-Santiago et al. 2006, Humeniuk et al. 2007), there is reason to think that its cytotoxic effects could vary with cellular oxygen levels. This could affect its efficacy as an agent for the treatment of solid tumours. We therefore examined the effect of oxygenation on the cytotoxicity of Aplidin.
Materials and methods
Radiation and drugs
Cell survival curves were determined using exponentially-growing monolayers irradiated with 250 kVp X-rays (15 mA, 2 mm Al equivalent filtration) produced by a Siemen's Stabilipan (New York, NY, USA) at a dose rate of 1.1 Gy/min. Radiation sterilised cells for the Bystander effect study were prepared from cell suspensions irradiated with 100 Gy of gamma rays from a Shepherd (San Fernando, CA, USA) Mark 1 137Cs irradiator at a dose rate of 12 Gy/min. Aplidin was provided by PharmaMar USA (Cambridge, MA. USA) as a powder and was dissolved in dimethyl sulfoximide (DMSO; JT Baker, Phillipsburg, NJ, USA) at a concentration of 1.8 mM (2 mg/ml), then diluted in DMSO to produce the concentrations used for experiments.
Cells
All experiments used EMT6 mouse mammary tumour cells (Subline EMT6 Rw). This cell line has been widely used to examine the effects of radiation on cells and solid tumours and to examine the modulation of cellular radiosensitivity by hypoxia, by chemical radiosensitisers and radioprotectors, and by cytotoxic anti-cancer drugs (Rockwell et al. 1977, 1992, 1997, 2009). The cells have also been widely used to examine the effects of hypoxia and low pH on the activity of anticancer drugs (Rockwell 1992, 2009). Cells were grown in Waymouth's medium (GIBCO, Carlsbad CA, USA) supplemented with 15% serum (Fetal plex™, Gemini, West Sacramento CA, USA) and antibiotics (GIBCO), exactly as described previously (Rockwell 1977, 2009). Cultures in exponential growth incubated at a temperature of 37°C in an atmosphere of 95% air/5% CO2 were used throughout these experiments unless specified otherwise.
Measurement of cell survival
Cell survival was measured using a clonogenic assay described in detail elsewhere (Rockwell 1977, 2009). Briefly, cells were suspended after treatment, washed thoroughly, twice, to remove residual drug and suspended using 0.5% trypsin (GIBCO). The suspended cells were counted using a Coulter Counter and plated at known densities into Petri dishes containing the Waymouth's medium described above; four replicate dishes were plated for each group. Cultures were incubated for two weeks to allow individual cells to grow into macroscopic colonies which were then fixed, stained, and counted. Untreated and vehicle-treated control cultures were included in each experiment. In most experiments, surviving fractions were calculated as the ratios of the plating efficiencies (PE = colonies per 100 cells plated) of treated cultures (PEtreated) and control (PEcontrol) cultures from the same experiment. For cultures incubated for 24 h in hypoxia or with drug, the cell numbers in the treated and control cultures at the end of treatment were different, probably as a result of cell death and/or inhibition of cell proliferation during treatment. In these experiments, cell survivals were corrected for the differences in the cell numbers, and are presented as the ‘Yield Corrected Surviving Fractions’ (Rockwell and Schulz 1984, Rockwell 2009), which were calculated as:
The Yield Corrected Surviving Fractions reflect both the lower cell numbers in the treated cultures and the lower colony forming ability of the cells present in these cultures.
Studies of the effect of acute severe anoxia on the cytotoxicity of Aplidin
Exponentially-growing EMT6 cell cultures in sealed glass flasks were made acutely hypoxic for 1 h at 37°C, by gassing with a humidified mixture of 95% N2, 5% CO2 containing, < 1 ppm O2, as described previously (Rockwell et al. 1992, 2009, Donnelly et al. 2004). Aplidin was added to the medium without compromising the hypoxia. Aerobic cultures were treated analogously with 95% air, 5% CO2. Two hours later, the medium was removed and the cells were washed twice, suspended, counted, diluted, and plated for colony formation.
Studies of the effect of prolonged moderate hypoxia on Aplidin toxicity
Cultures in plastic flasks were made moderately hypoxic by incubation in a glove box incubator (Ruskin model in vivo2 400. Bridgend, UK) that allows manipulation and incubation of cultures at 37°C under a well-controlled atmosphere of 1% oxygen, 92% N2, and 7% CO2. Cultures were incubated in moderate hypoxia for 2 h, then Aplidin was added to the culture medium, working within the incubator, in hypoxia. Aerobic cultures were treated analogously in 95% air, 5% CO2. Cells were treated with Aplidin for 24 h, then the Aplidin was removed and the cells were washed twice, suspended, counted, diluted, and plated to assay cell survival.
Studies of Aplidin in combination with radiation
These studies used exponentially-growing cultures in plastic Petri dishes. In one set of studies, cells were treated with 1 μM Aplidin for 2 h and were irradiated during the final minutes of the drug treatment. In other studies, cells were incubated with 1 μM Aplidin for a total of 24 h before, during and after irradiation, with radiation delivered 20 h after the addition of Aplidin. At the end of Aplidin treatment, the medium was removed and the cells were washed twice, suspended, counted, diluted, and plated for colony formation. Because Aplidin was dissolved in DMSO, which is a radioprotector, two irradiated control groups were used in these studies: One in normal cell culture medium and the other treated with DMSO at the concentration used to deliver Aplidin. Untreated cultures and cultures treated only with Aplidin or DMSO were also included in each experiment.
Bystander effects of Aplidin
Exponentially-growing cultures were treated either with 1 μM Aplidin or with DMSO for 24 h. The cultures were then washed twice and the cells were suspended, counted, spun down, and resuspended in cell culture medium at a concentration of 5 × 105 cells/ml. Both the Aplidin-treated and DMSO-treated cell suspensions were then irradiated with 100 Gy of 137Cs gamma rays, a dose sufficient to kill all of the cells. The radiation-sterilised cell suspensions were then diluted and plated into Petri dishes containing 5 ml of medium in concentrations ranging from 0–5 × 105 radiation-sterilised cells per dish. A total of 24 h later, untreated EMT6 cells, harvested from exponentially-growing cell cultures, were counted, diluted, and plated at low densities into the dishes that contained the radiation-sterilised cells. Cultures were then incubated for two weeks to allow the viable cells to grow into macroscopic colonies.
Results
Toxicity of Aplidin to aerobic and hypoxic cells
Short treatments (2 h) with graded doses of Aplidin were toxic to exponentially-growing EMT6 cells under normally aerated and severely hypoxic conditions (Figure 1). The survival of the cells decreased exponentially as the concentration of Aplidin increased. The response of cells to Aplidin under aerobic and severely hypoxic conditions was indistinguishable.
Figure 1.
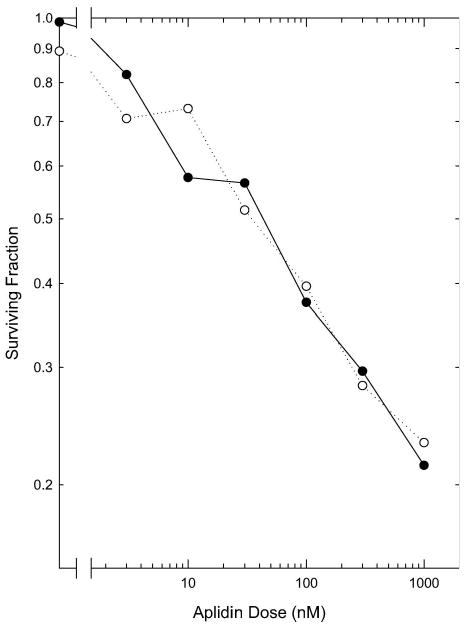
Cytotoxicity of a 2 h treatment with Aplidin. Exponentially-growing cells were treated with graded doses of Aplidin for 2 h under normal aeration (●) or severe hypoxia (○). Cell viability was assayed using a colony formation assay. Points are means from two independent experiments.
A 24 h treatment with Aplidin was more effective than the 2 h treatment under both aerobic and moderately hypoxic conditions (1% oxygen). The clonogenicity of the cells decreased exponentially as the dose of Aplidin increased (Figure 2). In addition, the number of cells in the cultures at the end of the 24 h treatment was significantly lower in the Aplidin-treated cultures; cell numbers decreased as the Aplidin dose increased (Figure 2). DMSO-treated and hypoxia-treated groups also had lower cell numbers than the aerobic controls. Incubation in moderate hypoxia (1% O2) throughout treatment did not alter the effects of Aplidin, as assayed by changes in the surviving fraction, cell yield, or yield corrected surviving fraction.
Figure 2.
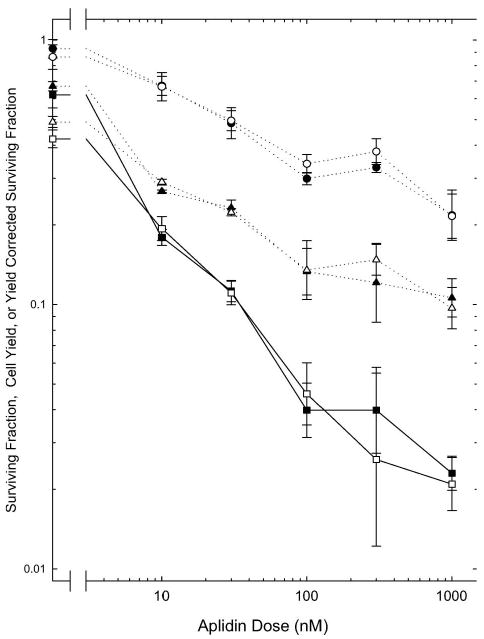
Effects of a 24 h treatment with Aplidin. Exponentially-growing cells were treated with Aplidin for 24 h under normal aeration (closed symbols) or moderate hypoxia (open symbols). Cell survival was assayed using a colony formation assay. Circles show the colony forming ability of the cells suspended at the end of the 24 h treatment. Triangles show the relative number of the cells present at the end of treatment, normalised to the untreated aerobic controls. The total contribution of both factors is shown by the Yield Corrected Surviving Fractions (squares, solid lines), calculated as described in Methods. Points are means ± SEM (Standard errors of the means) from three independent experiments.
Aplidin and radiation
The effect of a 2 h concomitant treatment with 1 μM Aplidin on the radiosensitivity of EMT6 cells is shown Figure 3. The survival curve for radiation alone is similar to that reported previously for this radioresistant cell line (Rockwell 1977, 1992, Rockwell and Schulz 1984, Rockwell 1997, Rockwell et al. 2009). Cells treated with DMSO, the vehicle used to dissolve the Aplidin, showed a slightly higher survival after high radiation doses than did cells treated only with radiation, as would be expected because DMSO is an extremely effective radical scavenger that acts as a radioprotector by decreasing the production of radiation-induced DNA damage. The survival curve for cells treated with Aplidin plus radiation is not significantly different from that of cells treated with radiation plus DMSO. Aplidin did not act as a radiosensitiser when given on this regimen; the toxicities of radiation plus Aplidin were strictly additive.
Figure 3.
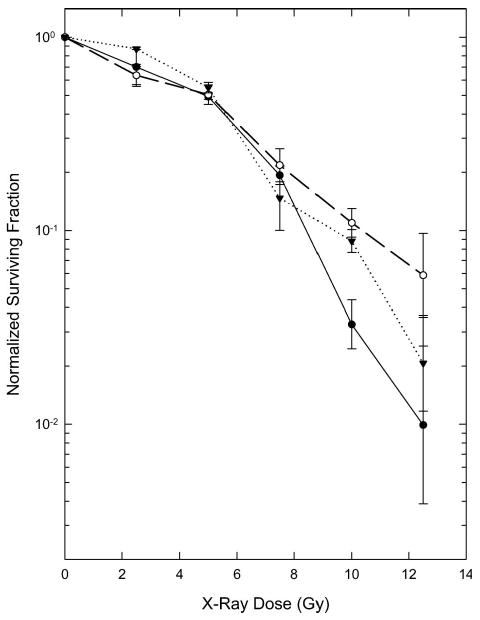
Cytotoxicity of radiation alone or in combination with 1 μM Aplidin for 2 h before and during irradiation. The survival curves for radiation in combination with Aplidin or DMSO were normalised to the surviving fraction of the unirradiated Aplidin-treated (0.20 ± 0.03) or DMSO-treated (1.07 ± 0.08) controls. ●: Radiation only. ○: DMSO plus Radiation. ▼: Aplidin plus Radiation. Points are means ± SEM from three independent experiments.
The effect of a 24 h treatment with 1 μM Aplidin on the radiosensitivity of EMT6 cells was examined in the experiments shown in Figure 4. Treatment with Aplidin alone produced marked cytotoxicity, reducing both the number of cells in the cultures at the end of treatment and the colony forming ability of the cells that were present. Findings from these experiments are therefore presented as Yield Corrected Surviving Fractions. The survival curve for Aplidin plus radiation has a shape similar to that for radiation alone, but offset to lower survival; reflecting the cytotoxicity of drug (Figure 4, left panel). When the survival curve for Aplidin plus radiation was corrected for drug toxicity by normalising the survival curve to the corresponding non-irradiated control group (Figure 4, right panel), the resulting curve was similar to those for radiation alone and radiation plus DMSO at low radiation doses, but diverged from these curves at high radiation doses. It was also noted during the analysis of data from these experiments that the number of colonies developing in cultures treated with 1 μM Aplidin plus high doses of radiation decreased as the number of cells plated for the colony formation assay increased. Because of this unusual finding, the surviving fractions shown on Figure 4 were determined using the colony counts in the dishes in which relatively low cell numbers were plated.
Figure 4.
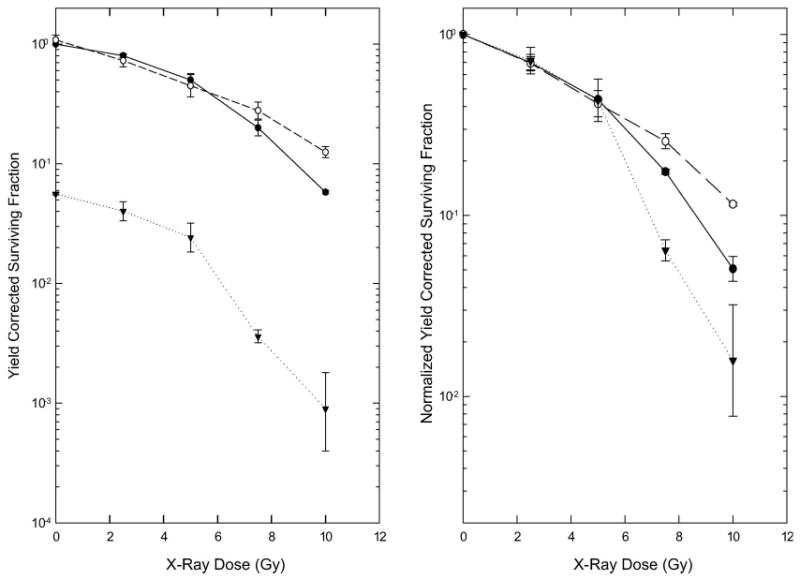
Cytotoxicity of radiation alone or in combination with a 24 h treatment with 1 μM Aplidin. Cultures were assayed for cell number and clonogenicity 24 h after addition of Aplidin (4 h after irradiation). The Left Panel shows the Yield Corrected Surviving Fraction, and therefore compares the total cytotoxicity in the three groups. On the Right Panel, the data for irradiated cultures have been normalised to the corresponding non-irradiated drug- or DMSO-treated control to facilitate comparison of the effect of radiation in the three groups. ●: Radiation only. ○: DMSO plus Radiation. ▼: Aplidin plus Radiation. Points are means ± SEM from three independent experiments. Note the difference in the scales of the Y axes in the two panels.
Bystander effects produced by Aplidin
Further experiments were performed to determine the basis for the unanticipated and unusual finding described above. In these experiments (Figure 5), exponentially-growing cultures were either treated with 1 μM Aplidin for 24 h or sham-treated with DMSO. The cultures were then washed twice, and the cells were suspended, counted, spun down, and resuspended at a concentration of 5 × 105 cells/ml and irradiated with 100 Gy, a dose sufficient to completely abolish the ability of the cells to form colonies. These radiation-sterilised cells were plated into Petri dishes at concentrations ranging from 5 × 103 to 5 × 105 cells per dish. Some 24 h later, untreated EMT6 cells, harvested from exponentially-growing cell cultures, were counted, diluted, and plated at low densities into the dishes containing the monolayers of radiation-sterilised cell. Cultures were then incubated for two weeks to allow these viable cells to grow into macroscopic colonies.
Figure 5.
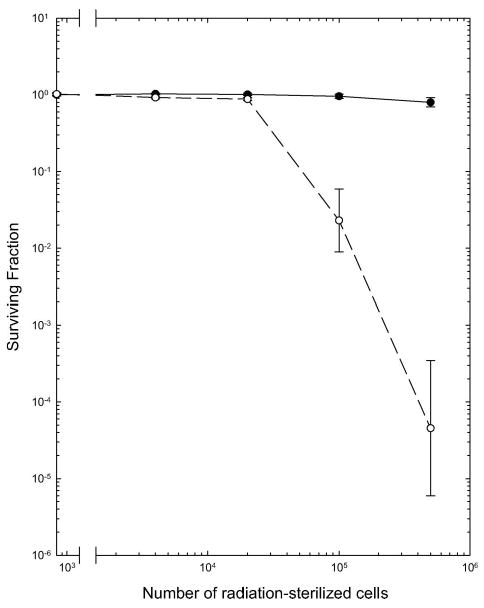
Effect of dying cells on the colony forming ability of untreated EMT6 cells co-cultured with them. Untreated, exponentially-growing EMT6 cells were plated onto monolayers initiated 24 h previously with graded numbers of radiation-sterilised cells that had been pretreated for 24 h with DMSO (●) or 1 μM Aplidin (○). Cultures were assayed for colony formation 14 days after plating. Surviving fractions were calculated relative to the PE of cells plated into dishes containing only cell culture medium. Points are means ± SEM from three independent experiments.
Radiation-sterilised, DMSO-treated cells did not alter the ability of the untreated EMT6 cells to form colonies at any dying cell concentration examined (Figure 5). This observation is in good agreement with the past studies performed during the optimisation of our cell culture assays for this cell line and with the extensive data from our past radiobiology studies with EMT6 cells (Rockwell and Schulz 1984, Rockwell 1977, 1992, 1997, 2009, Rockwell et al. and Grindey 1992, Donnelly et al. 2004). In marked contrast, Aplidin-pretreated, radiation-sterilised cells markedly inhibited the ability of the untreated EMT6 cells to form colonies. The viability of the untreated cells decreased as the number of dying Aplidin-treated cells in the dish increased. In addition, the colonies forming in cultures containing large numbers of dying Aplidin-treated cells were small, irregular and contained fewer cells than the colonies in control cultures or in cultures plated onto DMSO-treated, radiation-sterilised cells. These findings suggest that the dying Aplidin-treated cells produced a cytotoxic ‘Bystander effect’, which injured and killed viable cells co-cultured with them.
Discussion
The experiments reported here offer several insights into the effects of Aplidin that could be of value in planning for the use of this drug in the treatment of solid tumours and in considering the use of regimens combining this drug with radiotherapy.
First, neither acute severe hypoxia (< 1 ppm O2) nor chronic moderate hypoxia (1% O2) altered the cytotoxicity of Aplidin. Thus, although Aplidin is known to alter mitochondrial function and to result in the production of reactive oxygen species (ROS) when used to treat aerobic cells in cell culture (Biscardi et al. 2005, González-Santiago et al. 2006, Humeniuk et al. 2007), molecular oxygen is not required for its cytotoxicity. The fact that Aplidin retained its full activity under hypoxia is encouraging for its use against solid tumours. Solid malignancies contain large numbers of hypoxic cells; in fact, in some malignancies, the majority of the viable tumour cells are moderately or severely hypoxic. Hypoxic cells are resistant to radiation and to many commonly used anti-cancer drugs (Arbeit et al. 2006, Dewhirst 2009, Rockwell et al. 2009). The fact that Aplidin was found to be as toxic to moderately and severely hypoxic cells as to aerobic cells therefore makes this drug potentially valuable in the treatment of solid cancers.
Cell numbers in cultures treated with Aplidin, severe hypoxia or DMSO for 2 h were similar to those in control cultures, indicating that Aplidin did not induce rapid cell death. The lower cell numbers in the cultures treated with Aplidin for 24 h could reflect either the inhibition of cell proliferation by Aplidin or the death of some Aplidin-treated cells during the period of drug treatment; the assay used cannot distinguish between these two possibilities.
Our studies provided no evidence that a 2 h exposure to 1 μM Aplidin altered the radiation response of EMT6 cells. Experiments combining a 24 h treatment with Aplidin with radiation are more difficult to interpret. There are several mechanisms by which anticancer drugs can alter the response of cells to radiation. When present before irradiation, they can alter the rate of proliferation, the cell cycle distribution, or the physiology of the cells. When present during irradiation they can become involved in the chemical reactions that lead to the production of the DNA damage that kills irradiated cells. When present after irradiation they can modify the processes that lead to the repair of sublethal or potentially lethal DNA damage, which occurs during the first few hours after irradiation. To ensure that any of these possible mechanisms of interaction would be detected in these experiments, Aplidin was added to the cultures 20 h before irradiation and remained on the cultures during and for 4 h after irradiation. At low radiation doses, there were no significant differences between the survival curves for vehicle-treated cultures and Aplidin-treated cultures when the cytotoxicity of Aplidin was considered (Figure 4), suggesting that Aplidin and radiation have additive cytotoxicities. At high radiation doses, the data are statistically compatible with a greater than additive effect, which could reflect either a direct effect of Aplidin on cellular radiosensitivity or, more likely, a Bystander effect produced by dying Aplidin-treated cells during the colony formation assay.
In the clonogenic assay, increasing numbers of cells are plated for colony formation when the expected survival falls (a statistical necessity to ensure that adequate numbers of colonies form at low survivals). For control cultures, only 150 cells were plated in each dish. The surviving fractions after treatment with Aplidin plus radiation could not be predicted with certainty before the experiments. To ensure that either radioprotection by Aplidin or supra-additive interactions between Aplidin and radiation could be detected after high doses of radiation, four sets of dishes were actually plated, using 5-fold dilutions of cells, with the expectation that colony numbers in these dishes would likewise differ by 5-fold increments and that one set of dishes would contain optimal numbers of colonies for counting. This was not the case. Instead, the number of colonies per plated cell decreased as the number of plated cells increased. This did not occur in the groups receiving radiation or radiation plus DMSO.
Radiation sterilised EMT6 cells do not die immediately, or even rapidly, after irradiation. This reflects the mode of death in the irradiated cells. Although hematopoietic cells and a few other cell types die by apoptosis or other forms of interphase death soon after irradiation, most irradiated cells die in abortive mitoses, several cell cycles after irradiation (Elkind and Whitmore 1967, Chu et al. 2004). Radiation-sterilised EMT6 cells continue proliferating for one, two or even several cell cycles, producing several progeny, all of which will eventually die, days or weeks after irradiation. In heavily irradiated cultures, such as those in the experiments shown on Figures 3–5, cells will continue to die throughout the incubation for colony formation. Our data suggest that as dying cells treated previously with 1 μM Aplidin die and lyse they release a toxic moiety that is capable of killing neighbouring, previously uninjured cells. The complete absence of such cytotoxicity in cultures containing similar numbers of DMSO-treated, radiation-sterilised cells (Figure 5) proves that this is not simply a result of the presence of large numbers of dying cells or the release of some toxic product from cells killed by radiation. Our hypothesis is that cells treated for 24 h with 1 μM Aplidin sequester and retain Aplidin or an active metabolite of Aplidin after removal of the extracellular drug, and that this active moiety is slowly released as the treated cells die and lyse, is taken up by neighbouring cells, and produces toxic effects on these cells. Further studies would be needed to test this hypothesis.
This Bystander effect is interesting and unusual. Such effects are not seen with most anti-cancer drugs. Similar effects have been seen in two of our previous studies: Studies with high doses of tritiated thymidine, in which the radiolabeled nucleotide was released by dying cells, taken up by neighbouring cells and incorporated into the DNA of those Bystander cells, where it decayed and produced cytotoxicity (Rockwell et al. 1976) and studies with gemcytabine, in which Bystander cytotoxicity was ascribed to the release of the difluorodeoxycytodine by dying cells and uptake of this fraudulent nucleotide by neighbouring cells, resulting in toxicity (Rockwell and Grindey 1992). This Bystander effect of Aplidin could be useful in the treatment of solid tumours. Because the compromised perfusion within solid tumours prevents rapid efflux of drugs from the tumours, such an effect could result in the continued ‘slow release’ and prolonged presence of the active moiety within tumours during a course of treatment with fractionated radiotherapy, thereby increasing the killing of the tumour cells and the efficacy of the overall treatment.
Preclinical studies have shown the activity of Aplidin in a wide variety of human and mouse tumour cell lines and in numerous human tumour xenografts and mouse tumour models; these studies have supported the clinical use of this drug in cancer chemotherapy. Our findings suggest that regimens combining Aplidin and radiation may also be of value for the treatment of solid tumours, and encourage preclinical studies examining such regimens in animal model systems.
Acknowledgments
The authors thank Jacqueline Mendes for her assistance with the experiments reported here and Bettina Harris for her assistance with the preparation of the manuscript. We also thank PharmaMar for their support of this research and for providing the Aplidin for these studies. We thank Dr Enrique Alvarez for his discussions of these experiments and the results.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D. International Journal of Radiation Biology. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- Biscardi M, Caporale R, Balestri F, Gavazzi S, Jimeno J, Grossi A. VEGF inhibition and cytotoxic effect of aplidin in leukemia cell lines and cells from acute myeloid leukemia. Annals of Oncology. 2005;16:1667–1674. doi: 10.1093/annonc/mdi311. [DOI] [PubMed] [Google Scholar]
- Chu K, Teele N, Dewey MW, Albright N, Dewey WC. Computerized video time lapse study of cell cycle delay and arrest, mitotic catastrophe, apoptosis and clonogenic survival in irradiated 14-3-3σ and CDKN1A (p21) knockout cell lines. Radiation Research. 2004;162:270–286. doi: 10.1667/rr3221. [DOI] [PubMed] [Google Scholar]
- Donnelly ET, Kelley M, Rockwell S. Effects of RSR13 and oxygen on the cytotoxicity of cisplatin and carboplatin to EMT6 mouse mammary tumor cells in vitro and in vivo. Cancer Chemotherapy Pharmacology. 2004;53:43–50. doi: 10.1007/s00280-003-0715-8. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis, and oxidative stress: The Fialla Lecture. Radiation Research. 2009;172:653–665. doi: 10.1667/RR1926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MM, Whitmore GF. The radiobiology of cultured mammalian cells. New York: Gordon and Breach; 1967. [Google Scholar]
- Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. Journal of Biological Chemistry. 2005;280:11641–11647. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- García-Fernández LF, Reyes F, Sánchez-Puelles JM. The marine pharmacy: New antitumoral compounds from the sea. Pharmaceutical News. 2002;9:495–501. [Google Scholar]
- González-Santiago L, Alfonso P, Suárez Y, Núñez A, García-Fernández LF, Alvarez E, Muñoz A, Casal JI. Proteomic analysis of the resistance to Aplidin in human cancer cells. Journal of Proteome Research. 2007;6:1286–1294. doi: 10.1021/pr060430+. [DOI] [PubMed] [Google Scholar]
- González-Santiago L, Suárez Y, Zarich N, Muñoz-Alonso MJ, Cuadrado A, Martínez T, Goya L, Iradi A, Sáez-Tormo G, Maier JV, Moorthy A, Cato AC, Rojas JM, Muñoz A. Aplidin induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation. Cell Death Differentiation. 2006;13:1968–1981. doi: 10.1038/sj.cdd.4401898. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Menon LG, Mishra PJ, Saydam G, Longo-Sorbello GS, Elisseyeff Y, Lewis LD, Aracil M, Jimeno J, Bertino JR, Banerjee D. Aplidin synergizes with cytosine arabinoside: Functional relevance of mitochondria in Aplidin-induced cytotoxicity. Leucemia. 2007;21:2399–2405. doi: 10.1038/sj.leu.2404911. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Ocio EM, Pandiella A, Maiso P, Gasate C, Garayoa M, Vilanova D, Montero JC, Mitsiades N, McMullan CJ, Munsi NC, Hideshima T, Chauhan D, Aviles P, Otero G, Faircloth G. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Research. 2008;68:5216–5225. doi: 10.1158/0008-5472.CAN-07-5725. [DOI] [PubMed] [Google Scholar]
- Moneo V, Serelde BG, Leal JF, Blanco-Aparicio C, Diaz-Uriarte R, Aracil M, Tercero JC, Jimeno J, Carnero A. Levels of p27kip1 determine Aplidin sensitivity. Molecular Cancer Therapeutics. 2007;6:1310–1316. doi: 10.1158/1535-7163.MCT-06-0729. [DOI] [PubMed] [Google Scholar]
- Muñoz-Alonso MJ, González-Santiago L, Zarich N, Martínez T, Alvarez E, Rojas JM, Muñoz A. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-Jun NH2-terminal kinase activation in human melanoma cells. Journal of Pharmacology Experimental Therapeutics. 2008;324:1093–1101. doi: 10.1124/jpet.107.132662. [DOI] [PubMed] [Google Scholar]
- Rinehart KI. Antitumor compounds from tunicates. Medical Research Reviews. 2000;20:1–27. doi: 10.1002/(sici)1098-1128(200001)20:1<1::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Rockwell S, Frindel E, Tubiana M. A technique for determining the proportion of the clonogenic cells in S phase in EMT6 cell cultures and tumors. Cell Tissue Kinetics. 1976;9:313–323. doi: 10.1111/j.1365-2184.1976.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumor systems: New models for studying the response of tumors to therapy. Laboratory Animal Science. 1977;27:831–851. [PubMed] [Google Scholar]
- Rockwell S, Schulz RJ. Failure of 5-thio-D-glucose to alter cell survival in irradiated or unirradiated EMT6 tumors. Radiation Research. 1984;100:527–535. [PubMed] [Google Scholar]
- Rockwell S, Grindey GB. Effect of 2′, 2′-difluorodeoxycytidine on the viability and radiosensitivity of EMT6 cells in vitro. Oncology Research. 1992;4:151–155. [PubMed] [Google Scholar]
- Rockwell S. Use of hypoxia-directed drugs in the therapy of solid tumors. Seminars in Oncology. 1992;19:29–40. [PubMed] [Google Scholar]
- Rockwell S. Oxygen delivery: Implications for the biology and therapy of solid tumors. Oncology Research. 1997;9:383–390. [PubMed] [Google Scholar]
- Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Current Molecular Medicine. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell S. Tumor cell survival. In: Teicher BA, editor. Tumor models in cancer research. 2nd. New York: Humana Press; 2009. in press. [Google Scholar]
- Straight AM, Oakley K, Moores R, Bauer AJ, Patel A, Tuttle RM, Jimeno J, Francis GL. Aplidin reduces growth of anaplastic thyroid cancer xenografts and the expression of several angiogenic genes. Cancer Chemotherapy Pharmacology. 2006;57:7–14. doi: 10.1007/s00280-005-0014-7. [DOI] [PubMed] [Google Scholar]
- Suárez Y, González-Santiago L, Zarich N, Dávalos A, Aranda JF, Alonso MA, Lasunción MA, Rojas JM, Muñoz A. Plitidepsin cellular binding and Rac1/JNK pathway activation depend on membrane cholesterol content. Molecular Pharmacology. 2006;70:1654–1663. doi: 10.1124/mol.106.025569. [DOI] [PubMed] [Google Scholar]


