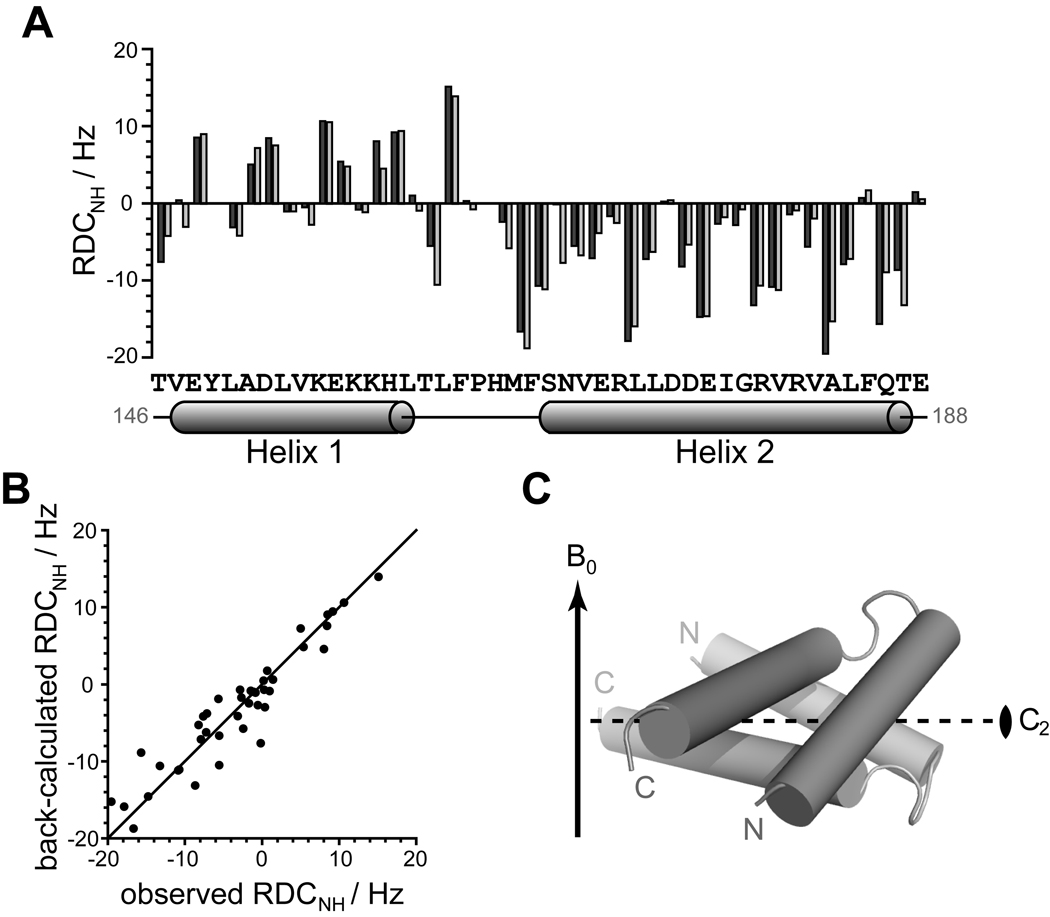
Figure 5. Experimental 1H-15N residual dipolar couplings (RDC) agree well with the Qua1 homodimer crystal structure.
(A) Experimental RDC obtained from aligning 15N-labeled Qua1 with Pf1 phage (dark gray) and back-calculated 1H-15N-RDCs from the crystal structure (light gray) plotted against protein sequence for the structural core of Qua1. The secondary structure according to the CS-Rosetta model is mapped onto the sequence. (B) Plotting of the back-calculated RDC from the crystal structure against the experimental values shows good correlation. (C) Alignment of the dimer model relative to the magnetic field B0 (indicated by an arrow) as determined by fitting the orientation of the Qua1 dimer crystal structure to the experimental RDC. The 2-fold symmetry axis (C2) of the homodimer is indicated by a dashed line.