Abstract
Background
Severe asthma causes the majority of asthma morbidity. Understanding mechanisms that contribute to the development of severe disease is important.
Objective
The goal of the Severe Asthma Research Program is to identify and characterize subjects with severe asthma to understand pathophysiologic mechanisms in severe asthma.
Methods
We performed a comprehensive phenotypic characterization (questionnaires, atopy and pulmonary function testing, phlebotomy, exhaled nitric oxide) in subjects with severe and not severe asthma.
Results
A total of 438 subjects with asthma were studied (204 severe, 70 moderate, 164 mild). Severe subjects with asthma were older with longer disease duration (P < .0001), more daily symptoms, intense urgent health care utilization, sinusitis, and pneumonia (P ≤ .0001). Lung function was lower in severe asthma with marked bronchodilator reversibility (P < .001). The severe group had less atopy by skin tests (P = .0007), but blood eosinophils, IgE, and exhaled nitric oxide levels did not differentiate disease severity. A reduced FEV1, history of pneumonia, and fewer positive skin tests were risk factors for severe disease. Early disease onset (age < 12 years) in severe asthma was associated with longer disease duration (P < .0001) and more urgent health care, especially intensive care (P = .002). Later disease onset (age ≥ 12 years) was associated with lower lung function and sinopulmonary infections (P ≤ .02).
Conclusion
Severe asthma is characterized by abnormal lung function that is responsive to bronchodilators, a history of sinopulmonary infections, persistent symptoms, and increased health care utilization.
Clinical implications
Lung function abnormalities in severe asthma are reversible in most patients, and pneumonia is a risk factor for the development of severe disease.
Keywords: Severe asthma, definition, bronchodilator response, pathophysiology, phenotype, pneumonia
Severe asthma accounts for only 5% to 10% of patients with asthma, but it accounts for a considerable portion of the health care costs associated with this disease.1–3 Severe asthma remains poorly understood physiologically and pathologically.4,5 Patients with severe asthma are particularly difficult to treat, with current therapies incompletely controlling symptoms and even intensive treatment having little effect on health care utilization (HCU).6,7
In 2000, the National Heart, Lung, and Blood Institute (NHLBI) sponsored a workshop on severe asthma to assess current knowledge of biologic mechanisms contributing to the development of severe disease and propose directions for future investigations.4 In response to this workshop, NHLBI funded the Severe Asthma Research Program (SARP), consisting of 9 sites in the United States and 1 in the United Kingdom. A key goal of SARP was to identify and characterize a large number of subjects with severe asthma.
To identify subjects with severe and symptomatic asthma despite appropriate treatment, we used the definition of severe asthma developed by an American Thoracic Society (ATS) workshop on refractory asthma.8 This definition requires signs of ongoing poor asthma control (daily symptoms, additional medication use, high HCU, abnormal lung function) despite treatment with high doses of corticosteroids. Additional subjects who did not fulfill the ATS criteria for severe asthma were evaluated as a reference group. These not severe asthma subjects represented the spectrum of mild to moderate disease and were not preselected to represent either a very mild or well controlled group of subjects with asthma. All subjects underwent a comprehensive phenotypic characterization that included standardized questionnaires, pulmonary function testing, atopy evaluations, measurement of exhaled nitric oxide, and collection of blood.
This article describes the SARP cohort in detail, emphasizing clinical characteristics, HCU, and pulmonary function of the subjects with severe asthma. We analyzed the overall contribution of several factors that have been previously associated with severe asthma, including atopy, age of disease onset, duration of disease, and comorbidities.9–11
METHODS
After establishing standard operating procedures, including a review by an independent Data Safety Monitoring Board and approval by the Institutional Review Boards at each site, subjects underwent a comprehensive phenotypic characterization.
Subjects
Subjects included in this analysis provided written informed consent and completed clinical questionnaires and baseline spirometry. All subjects were nonsmokers (<5 pack-years of tobacco use). Subjects with asthma of all ages who met the ATS workshop definition of severe asthma were recruited from the subspecialty clinics of SARP investigators.8 This definition of severe asthma requires a combination of 1 of 2 major and 2 of 7 minor criteria and identifies subjects with poor asthma control despite treatment with high doses of corticosteroids (Table I).
TABLE I.
| Major criteria (need ≥1) |
| Treatment with continuous or near continuous (≥50% of year) OCSs |
| Requirement for treatment with high-dose ICSs |
| Minor criteria (need ≥2) |
| Requirement for additional daily treatment with a controller medication (eg, LABA, theophylline, or leukotriene antagonist) |
| Asthma symptoms requiring SABA use on a daily or near-daily basis |
| Persistent airway obstruction (FEV1 < 80% predicted, diurnal peak expiratory flow variability > 20%) |
| One or more urgent care visits for asthma per year |
| Three or more oral steroid bursts per year |
| Prompt deterioration with a ≤25% reduction in oral or inhaled corticosteroid dose |
| Near-fatal asthma event in the past |
Requires that other conditions have been excluded, exacerbating factors have been treated, and patient is generally compliant.
An additional group of subjects with asthma that did not meet the criteria for severe asthma (not severe) was studied. The not severe group represented a spectrum of asthma severity from mild to moderate disease. For this analysis, the asthma severity of the not severe subjects was classified in a post hoc manner. These definitions of mild and moderate asthma were based primarily on lung function and the use of inhaled corticosteroids (ICSs), a classification scheme that has been used to define asthma severity in national and international guidelines.12–14 Asthma symptoms, exacerbations, and HCU were not used to define the not severe groups to allow differentiation of these endpoints among the 3 groups. Mild asthma was defined as a prebronchodilator FEV1 ≥ 80% predicted in subjects treated with either no or low to moderate dose ICS (<880 µg fluticasone or equivalent). Moderate asthma was defined as a prebronchodilator FEV1 < 80% predicted in subjects on low to moderate dose ICS.
Questionnaires
Clinical staff administered comprehensive questionnaires to all subjects. Questionnaires included general information on demographics, medical comorbidities, smoking, and family history. Subjects were asked to gauge the frequency of asthma symptoms (cough, sputum, chest tightness, wheeze, shortness of breath, nocturnal symptoms) over the past 3 months to characterize persistent asthma symptoms. Asthma exacerbations and HCU were assessed by reported history of emergency department (ED) visits and hospital or intensive care unit (ICU) admissions, as well as near fatal events that had occurred in the past 12 months or ever in their lifetime.
Pulmonary function
Pulmonary function testing was performed according to ATS guidelines.15 Subjects were instructed to withhold bronchodilators before methacholine bronchoprovocation and baseline spirometry if their asthma symptoms permitted (4 hours for short-acting β-agonists [SABAs], 12 hours for long-acting β-agonists [LABAs]). The FEV1 from an appropriate medication withhold day was used as the subject’s baseline FEV1 for analysis. Subjects with low lung function (FEV1 < 55% predicted) were excluded from methacholine testing because of safety concerns (Asthma Clinical Research Network IND #46,881). Maximal response to SABA was assessed after the administration of 4, 6, and 8 puffs of albuterol (360, 540, 720 µg albuterol). Results are expressed as the best FEV1 and maximal % change in FEV1.
Assessment of atopy and inflammation
Skin prick testing to 14 common aeroallergens was performed. Blood was collected for measurement of total serum IgE and a complete blood count. Exhaled nitric oxide (FeNO) was measured online by chemiluminescence at a constant expiratory flow (50 mL/s), consistent with published guidelines.16
Statistical analysis
Data are presented as means ± SDs. Total IgE (adjusted for age and sex), blood eosinophils, exhaled nitric oxide levels, and PC20 methacholine data were log-transformed to achieve a normal distribution. Continuous variables were analyzed by ANOVA, categorical variables by χ2 tests. If initial analyses revealed a significant difference among the 3 groups (mild, moderate, and severe), contrasts were used to identify specific pairwise differences. The false discovery rate procedure was used to control for multiple comparisons, and only those passing this test are reported as statistically significant.17 Both single and multiple variable approaches were used to define characteristics predictive of or associated with severe asthma. Variables that achieved an overall P value for differences between groups ≤ .1 were analyzed in a backward logistic regression model. Variables with a P < .05 were considered significant predictors of asthma severity.
RESULTS
Classification of disease severity
From August 2003 to May 16, 2005, the network enrolled 204 subjects with severe asthma, 70 subjects with moderate asthma receiving ICS, and 164 subjects with mild asthma (94 of whom were on ICS). Despite treatment with high doses of corticosteroids, the subjects with severe asthma had an average of 4 to 5 total minor criteria. Nearly all subjects with severe asthma were using a second controller medication, 78% had persistent airflow obstruction, and more than half were having frequent exacerbations that required urgent physician visits or treatment with oral corticosteroids (OCSs) during the past year (Table II). In contrast, only 2 minor criteria reached >50% frequency in the mild and moderate groups: the use of a second controller medication (typically a LABA/ICS combination) and worsening of symptoms with a decrease in corticosteroid dose. Interpretation of daily SABA use was confounded by subject report of prophylactic administration of albuterol before exercise as daily use. Although the minor criteria that connote the frequency or severity of asthma exacerbations were present in the mild and moderate groups, they were much less frequent; the history of 3 or more exacerbations requiring treatment with OCSs in the previous year or a previous history of endotracheal intubation best discriminated severe asthma from milder disease.
TABLE II.
Frequency of ATS severity8 criteria by disease severity
| Mild (n = 164) | Moderate (n = 70) | Severe (n = 204) | P value | |
|---|---|---|---|---|
| Major criteria | ||||
| OCSs for ≥50% of year | 0.6% | 0% | 32% | ND |
| High-dose ICSs | 0% | 0% | 98% | ND |
| Minor criteria | ||||
| Daily 2nd controller medication | 52% | 79% | 94% | <.0001* |
| Daily SABA‡ | 27% | 44% | 75% | <.0001* |
| Persistent airflow obstruction§ | 0% | 100% | 78% | ND |
| ≥1 urgent care visits/y | 16% | 31% | 54% | <.0001* |
| ≥3 OCS bursts/y | 5% | 13% | 54% | <.0001† |
| Deterioration with reduced corticosteroids | 32% | 60% | 78% | <.0001* |
| Near-fatal event in the past | 4% | 6% | 23% | <.0001† |
ND, Not determined.
Three-way comparison significant; all groups are different.
Three-way comparison, significant because of differences between severe vs mild and moderate.
Includes subject report of SABA prophylactic before exercise.
Baseline FEV1 ≤ 80%; measurement with bronchodilator withhold; ATS definition does not require withhold of bronchodilators.
Subject demographics
Only subjects ≥ 12 years of age at enrollment were included in the current analysis on the basis of previous reports of differences in childhood and adult asthma and the relatively few subjects < 12 years of age in the cohort (15 not severe, 14 severe).6,9 There was no difference in reported age of asthma onset among the groups (Table III). Subjects with severe asthma were older and duration of disease was longest in this group. There were more women in each severity group, but there was no difference in race or sex distribution among the groups. Although the use of ICS in each group reflects the definitions used to classify severity, there were other differences in asthma medications among the 3 groups (Table III). The majority (75%) of the subjects receiving ICS were on LABA/ICS combinations. Fewer than 10% of subjects were undergoing immunotherapy, and this treatment was not associated with asthma severity.
TABLE III.
Subject demographics and medication use
| Mild (n = 164) | Moderate (n = 70) | Severe (n = 204) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Current age (y) | 31 ± 12 | 38 ± 12 | 41 ± 13 | <.00001‡ |
| Age of asthma onset (y) | 15 ± 13 | 18 ± 15 | 16 ± 16 | .37 |
| Asthma duration (y) | 17 ± 11 | 20 ± 14 | 25 ± 14 | <.0001* |
| Sex (% female) | 72% | 56% | 64% | .05 |
| Race (% white/African American/other) | 69/25/6 | 66/29/5 | 67/26/7 | .92 |
| Asthma medications | ||||
| ICSs‖ | 58% | 100% | 98% | <.0001‡ |
| OCSs‖ | 0% | 0% | 32% | ND |
| LABAs§ | 48% | 80% | 89% | .001* |
| Leukotriene modifiers | 22% | 26% | 51% | <.0001† |
| Omalizumab | 0.1% | 0% | 12% | <.0001† |
| Theophyllines | 0% | 4% | 18% | <.0001* |
| Anticholinergic agents | 4% | 6% | 20% | <.0001† |
ND, Not determined.
Three-way comparison significant; all groups are different.
Three-way comparison, significant because of differences between severe vs mild and moderate.
Three-way comparison, significant because of differences between mild vs moderate and severe.
No subjects were on LABA monotherapy.
ICS/OCS use was part of the definition of disease severity.
Asthma symptoms and HCU
The frequency of all reported symptoms increased with disease severity. Cough (42% subjects) and shortness of breath (47%) were the most frequent daily symptoms in the severe group, but > 34% also reported daily nocturnal symptoms, wheezing, and chest tightness (P < .0001). Lower baseline FEV1 was associated with increasing frequency of shortness of breath in the severe group (P < .0001). In contrast, the majority of mild and moderate subjects reported less than monthly cough (51% and 54%, respectively) and nocturnal symptoms (69%, 60%). Wheezing and shortness of breath were more frequent in the moderate group compared with the mild group; 44% reported weekly wheeze and 41% weekly shortness of breath. Surprisingly, a small number of subjects in the mild group reported daily cough (n = 26) and shortness of breath (n = 23), but there was discordance with reported daily albuterol use; 74% of mild subjects who reported daily cough did not report daily albuterol. Conversely, 25% of mild subjects who reported daily albuterol use reported less than monthly symptoms. Concordance improved as disease severity worsened; the majority of severe subjects (>72%) who reported daily symptoms also reported daily albuterol use.
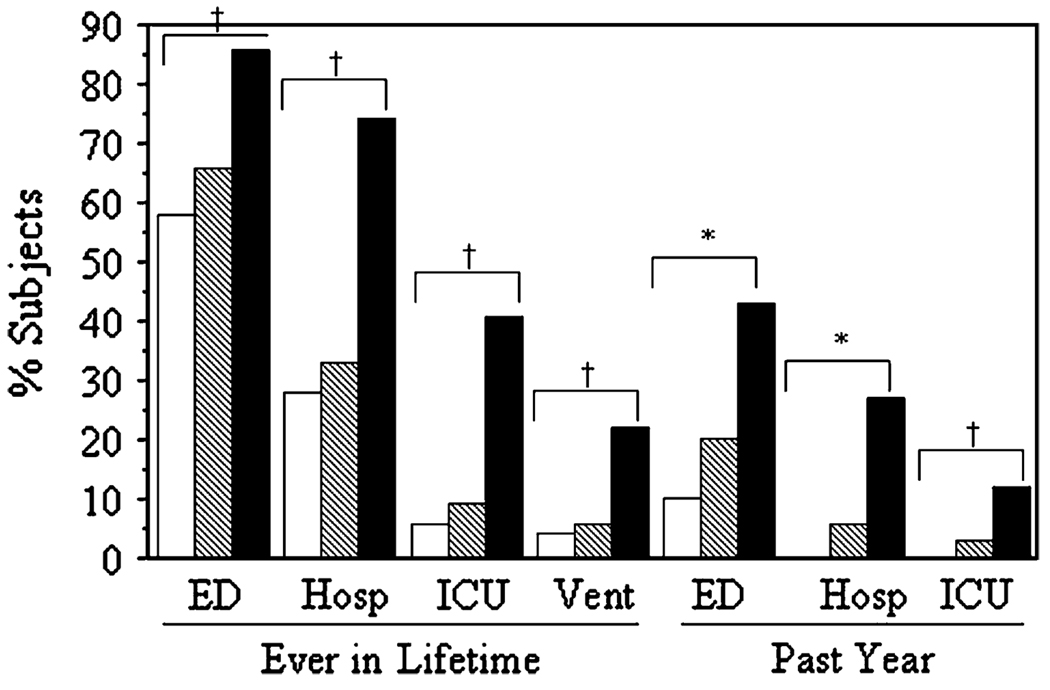
Urgent HCU was more common and more frequent in severe asthma, with increased ED visits, hospitalizations, and ICU stays ever and within the past 12 months (Fig 1). Although a history of an ED visit was more frequent in severe asthma, 58% to 66% of subjects with mild and moderate asthma had been to the ED ever for asthma exacerbations. In contrast, more expensive and intensive health care (hospitalization, ICU admission) was more characteristic of severe asthma. In the severe group, different daily symptoms were associated with type of urgent HCU: daily cough or chest tightness with ED visits and hospitalizations (odds ratio [OR], 3.99; CI, 1.76–9.06; P < .001; and OR, 2.15; CI, 1.15–4.02; P < .02, respectively), and weekly-daily nighttime awakenings with ICU events (OR, 2.19; CI, 1.11–4.34; P < .03). Interestingly, daily shortness of breath was associated with lower baseline FEV1 (P < .0001), but not urgent HCU.
FIG 1.
Urgent HCU increased with disease severity. Data are shown as lifetime (ever) events and visits in the past year. Hosp, Inpatient hospitalizations; Vent, need for mechanical ventilation. White bars, mild (n = 164); hatched bars, moderate; black bars, severe asthma. *P < .0001, all groups are different from each other; †P < .0001, mild and moderate groups are similar, but different from severe group.
Pulmonary function
The use of prebronchodilator baseline FEV1 to classify asthma severity led to similar mean FEV1 in the moderate and severe groups (Table IV). The distribution of FEV1 % predicted, however, was different in the 2 groups; 47% of the subjects with severe asthma had a baseline FEV1 < 60% predicted, compared with 18% in the moderate group. All groups showed improvement in airflow obstruction with bronchodilator testing (2–8 puffs of albuterol). Traditional reversibility testing (2 puffs albuterol) was a less sensitive test to assess bronchodilator responsiveness than maximal bronchodilator testing (4–8 puffs); 51% of severe subjects did not have a >12% improvement in FEV1 after 2 puffs of albuterol, while only 39% did not have >12% improvement with higher doses of albuterol. The magnitude of reversibility correlated with the level of baseline airway obstruction (r = 0.37; P < .0001), not disease severity per se. Only 30 severe subjects with a baseline FEV1 < 80% showed < 12% improvement with bronchodilators. There was less bronchial responsiveness to methacholine in the mild group compared with those with moderate to severe disease. There was no statistical difference in PC20 between the moderate and severe groups, but these data are difficult to interpret as a result of the exclusion of many subjects from methacholine testing because of a pretest FEV1 < 55% (66% of the moderate group and 43% of the severe group underwent methacholine bronchoprovocation).
TABLE IV.
Clinical characteristics of subjects
| Mild | n | Moderate | n | Severe | n | P value | |
|---|---|---|---|---|---|---|---|
| Baseline lung function | 164 | 70 | 204 | ||||
| FEV1 % predicted | 94 ± 11 | 66 ± 11 | 62 ± 22 | <.0001‡ | |||
| FVC % predicted | 100 ± 12 | 81 ± 13 | 77 ± 20 | <.0001* | |||
| FEV1/FVC (%) | 80 ± 7 | 67 ± 10 | 65 ± 13 | <.0001‡ | |||
| Best lung function | 157 | 60 | 185 | ||||
| FEV1 % predicted | 102 ± 11 | 79 ± 12 | 77 ± 21 | <.0001‡ | |||
| FVC % predicted | 103 ± 13 | 91 ± 14 | 91 ± 18 | <.0001‡ | |||
| Maximal % change in FEV1 | 9 ± 7 | 20 ± 16 | 20 ± 24 | <.0001‡ | |||
| Methacholine PC20 (log mg/mL) | .24 ± .62 | 133 | −.11 ± .54 | 46 | −.06 ± .70 | 87 | .0002‡ |
| FeNO (ppb) | 42 ± 48 | 120 | 45 ± 39 | 55 | 40 ± 38 | 135 | .72 |
| Blood eosinophils (log) | −.72 ± .42 | 151 | −.63 ± .46 | 63 | −.75 ± .51 | 180 | .19 |
| Total serum IgE (log) | 2.0 ± .75 | 151 | 2.1 ± .63 | 63 | 2.0 ± .76 | 159 | .44 |
| ≥1 positive skin test (%) | 85% | 164 | 87% | 70 | 71% | 204 | .0007† |
Three-way comparison significant; all groups are different.
Three-way comparison, significant because of differences between severe vs mild and moderate.
Three-way comparison, significant because of differences between mild vs moderate and severe.
Measures of atopy
Fewer subjects with severe asthma had positive skin prick tests (Table IV). The number of positive skin test responses in those who were reactive, however, was not different among the groups (mean number positive prick tests, 4.5 mild, 4.3 moderate, 4.4 severe; P = .86). There was no association between the use of OCSs and lack of skin test responses in the severe group (P = .32). In contrast with atopy, there were no statistically significant differences in blood eosinophil counts or total serum IgE levels among the groups.
Exhaled nitric oxide
Level of FeNO did not differentiate the mild, moderate, and severe groups. There was a wide range of values within each group (mild, 5–317 ppb; moderate, 7–169 ppb; severe, 4–197 ppb). There were positive correlations between FeNO and number of positive skin test responses (r = 0.23; P < .0001), blood eosinophils (r = 0.29; P < .0001), and serum IgE (r = 0.32; P < .0001) in the entire cohort, but these associations were not present when the severe group was analyzed separately (P > .15).
Comorbid conditions
The overall prevalence of reported remote tobacco use (<5 pack-years) was not different between the groups (12% to 18%; P = .21). Aspirin sensitivity was more common in the severe group (P = .01). Gastroesophageal reflux (GERD) and a history of sinopulmonary infections were reported more often in the severe group; 41% of the severe subjects reported GERD (vs 12% to 16% in the not severe groups; P < .0001), 54% a history of sinusitis (vs 33% to 37%; P = .0001), and 63% a history of pneumonia (vs 35% to 36%; P < .0001). Of the severe group, 27% reported sinus disease of such severity as to require surgical intervention in the past (vs 10% to 11% in the not severe groups; P < .0001).
Analysis of the multiple factors contributing to asthma severity
Univariate analysis was performed on 63 potential variables; 39 achieved a P value < .1 for differences across the groups and were then analyzed in a backward regression model. Five variables independently increased the likelihood that a subject would be classified with severe asthma: (1) prebronchodilator FEV1 % predicted with a 36% increase in risk of being classified as severe asthma for every 5% fall in FEV1 (P < .0001), (2) history of pneumonia (OR, 3.30; CI, 1.92–5.69; P < .0001), (3) lower numbers of blood basophils (OR, 2.55; CI, 1.46–4.47; P = .001), (4) asthma symptoms during routine physical activities (OR, 2.28;CI, 1.25–4.15; P = .007), and (5) lower numbers of (1) allergy skin test reactions (OR, 1.11; CI, 1.00–1.22; P = .04).
Effect of age of onset on the severe asthma phenotype
On the basis of the mean age of puberty (11 for girls, 12 for boys) and previous publications, 12 years of age was used to define early-onset (onset < 12 years) as opposed to late-onset (onset ≥ 12 years) asthma.10 Subjects with late-onset severe asthma were older but had a significantly shorter duration of disease than those with early-onset asthma (Table V). Pulmonary function was lower in late-onset asthma despite this shorter duration of disease, although this reached significance only for forced vital capacity (FVC) % predicted. The late-onset group appeared to have less bronchial hyperresponsiveness (P = .003), but less than half of subjects in this group were able to undergo methacholine challenge because of prestudy FEV1 < 55% predicted. There was no difference in the percentage of subjects with positive skin test responses between the groups (74% vs 67%; P = .36, respectively), but subjects with early-onset asthma had more positive skin tests (mean positive tests, 5.0 vs 3.7; P = .002) and more allergic asthma symptoms produced by triggers such as cats (P = .0002). Asthma symptoms, smoking history, reported aspirin sensitivity, and GERD symptoms were similar between the groups (data not shown), but more subjects with late-onset asthma reported a history of sinusitis (45% vs 67%; P = .003) and pneumonia (28% vs 45%; P = .02). Measures of self-reported lifelong ever HCU were greater in the subjects with early-onset asthma (92% vs 81% ED, P = .03; 84% vs 64% hospitalized, P = .002; 54% vs 31% ICU, P = .002; 29% vs 17% ventilator, P = .07), but there was no difference in these measures in the 12 months before entry (data not shown), suggesting that this increased lifetime frequency may be secondary to longer disease duration in the early-onset group.
TABLE V.
Clinical characteristics of severe asthma based on age of asthma onset
| Onset < 12 years (n = 100) | Onset ≥12 years (n = 104) | P value | |
|---|---|---|---|
| Current age (y) | 34 ± 13 | 48 ± 10 | <.0001 |
| Asthma duration (y) | 32 ± 13 | 19 ± 11 | <.0001 |
| Sex (% female) | 60% | 68% | .24 |
| Race (% white/African American/other) | 61/30/9 | 72/21/7 | .25 |
| Baseline lung function | |||
| FEV1 % predicted | 64 ± 21 | 59 ± 21 | .63 |
| FVC % predicted | 81 ± 21 | 73 ± 19 | .004 |
| FEV1/FVC % | .65 ± .14 | .64 ± .13 | .50 |
| Maximal % change in FEV1 | 20 ± 20 | 21 ± 27 | .77 |
| FeNO (ppb) | 38 ± 35 | 41 ± 41 | .69 |
| Blood eosinophils (log) | −.75 ± .47 | −.76 ± .54 | .84 |
| Total serum IgE (log) | 2.2 ± .69 | 1.9 ± .79 | .19 |
DISCUSSION
The overall goal of SARP is to investigate prospectively subjects with severe asthma to understand better the pathophysiologic and biologic mechanisms that result in this level of disease severity. This article describes the clinical and physiologic characteristics of the SARP cohort that consists of more than 400 subjects and includes the largest group of comprehensively characterized subjects with severe asthma published to date. This study presents a unique opportunity to confirm results of previous smaller studies while identifying new characteristics of severe asthma.
There is no gold standard for the assessment of disease severity in asthma.18 Studies on severe asthma have used a variety of definitions for severe disease that differ in their requirement for frequency and severity of symptoms and exacerbations, as well as dose of corticosteroids.19 We chose to use the ATS workshop definition of severe asthma to identify subjects with severe asthma for SARP. This definition was developed by consensus and is characterized by varying mixtures of persistent symptoms, complex medication needs, abnormal lung function, and a need for urgent health care evaluation despite appropriate treatment with high doses of ICSs or OCSs.8 This approach permitted prospective assessment of the importance of the individual components of the ATS definition.
We also studied a large number of subjects with not severe asthma who did not meet criteria for severe disease to have a reference group for comparison. These control subjects with asthma were not preselected to be the most mild or best controlled subjects with asthma, but rather represented a continuum of disease severity from mild to moderate asthma. To allow comparison of the severe asthma group with this spectrum of less severe disease, asthma severity in the not severe group was categorized post hoc. The assessment of disease severity in asthma is difficult, and the best method to assign disease severity in this group was unclear.15,18 Consideration was given to both the National Asthma Education and Prevention Program (NAEPP) and Global Initiative for Asthma (GINA) guidelines, as well as the use of physician global assessment.7,12–14,18,20 The NAEPP guidelines assess disease severity on the basis of frequency of symptoms and measures of lung function before treatment for asthma.12,13 Because only 16% of our subjects were not on controller medications, the NAEPP guidelines could not be applied to the SARP cohort. The GINA guidelines likewise utilize a classification scheme that includes lung function and symptoms to assess disease severity, but has included medication use in the past.14 Neither guidelines, however, incorporate measures of HCU. We chose to classify disease severity in the control subjects with not severe asthma by use of ICSs and lung function (FEV1), similar to the GINA guidelines, but without the use of HCU and/or symptoms, because we wanted to evaluate the occurrence of these events in severe asthma compared with not severe asthma. Despite this relatively loose definition of mild and moderate not severe asthma, comparison of these subjects with subjects with severe asthma revealed marked and widespread differences in symptoms, HCU, comorbidities, and immunopathologic factors. It is likely that these differences would have been more profound if the not severe group had been more rigidly selected for medication use, symptoms, and HCU.
We identified and characterized 204 subjects with severe asthma in SARP. Nearly 95% of the subjects with severe asthma were treated with a second long-term controller, the majority were on a third controller medication, and 12% were treated with anti-IgE therapy. This use of multiple controller medications in addition to high doses of corticosteroids implies that these subjects were on appropriate maximal therapy for asthma, yet they remained symptomatic and required high levels of HCU. Analysis of the 7 minor ATS criteria showed that the criteria that best discriminated severe from mild and moderate asthma were frequent (>3 OCS bursts in the previous year) and severe (history of intubation) asthma exacerbations.
The most frequently reported symptoms in severe asthma were cough and shortness of breath, not wheeze or chest tightness. Daily cough, chest tightness, and nighttime symptoms were associated with higher urgent health care requirements, whereas shortness of breath was related to lower FEV1. These distinctive symptoms should be specifically monitored as part of overall clinical care because the character of daily symptoms may identify patients at risk for persistent lung function abnormalities and/or adverse health outcomes.
Although the high frequency and intensity of HCU differentiated severe from milder asthma, an additional important finding in this study was that a substantial percentage of subjects with mild and moderate asthma, most treated with low to moderate dose ICS, had utilized emergency care for asthma exacerbations. Nearly 66% of subjects with mild and moderate asthma reported a lifetime history of ED visits. These data suggest that exacerbations requiring emergency treatment do occur in milder asthma (at least once), but these exacerbations likely resolve with therapy without leading to hospitalizations, and occur less often. In contrast, the majority of subjects with severe asthma reported a previous hospitalization for an asthma exacerbation, and more than 40% had a history of an ICU admission; 12% had been in the ICU in the previous year. These findings support previous studies that identify a history of a severe exacerbation as one of the strongest risk factors for additional severe exacerbation or near-fatal event.21–24
Low lung function is not a required criterion in the ATS definition of severe asthma, but 80% of severe subjects had persistent airflow obstruction (FEV1 < 80%), and nearly half had a baseline FEV1 < 60% when bronchodilators were withheld before spirometry. Reversibility testing using high doses of albuterol (6–8 puffs), however, showed more than 12% improvement in FEV1 in the majority (61%) of all severe subjects, even though they had not withheld their bronchodilators before testing. It is important to note that the magnitude of the response to bronchodilators correlated primarily with baseline FEV1; there was no difference in the degree of reversibility in the moderate and severe groups when matched for FEV1. Only 9% of the subjects with severe asthma with a baseline FEV1 < 60% showed no response to albuterol, regardless of dose, suggesting that the majority of subjects with severe asthma with low baseline lung function do not have irreversible airflow limitation characteristic of chronic obstructive pulmonary disease.25 The presence of significant reversibility in the severe asthma group is highlighted by the fact that whereas low FEV1 was a strong independent risk factor for severe asthma by logistic regression, low reversibility was not.
Fewer subjects with severe asthma had positive skin prick tests to 1 or more allergens. In addition, fewer positive skin test responses were an independent risk factor for severe disease by logistic regression. Although it is possible that skin test responses were suppressed by high doses of corticosteroids (oral or inhaled), the stability of allergen skin test responses despite chronic administration of OCSs has been previously reported,26 and there was no association between lack of skin test responses and treatment with OCSs. These findings support other reports of reduced allergic responses in severe asthma compared with those with milder disease.19 There was no difference in serum IgE levels or blood eosinophils among the 3 groups, but lower blood basophils were an independent risk factor for severe disease (OR, 2.55). The possible role of basophils in severe asthma warrants further investigation.
Exhaled nitric oxide level was not associated with disease severity in our study. Further, it was not associated with blood eosinophils, serum IgE, or treatment with OCSs in the patients with severe asthma. Previous reports suggest that determinants of FeNO levels are complex; although they often reflect eosinophilic airway inflammation, they can also reflect airway metabolism of superoxide, arginine, S-nitrosoglutathione, and other compounds.19,27–33 Our data suggest that subjects with severe asthma do not uniformly fall into the high FeNO-atopic phenotype; further, that clinical interpretation of FeNO levels may be complicated in severe asthma.
Aspirin sensitivity, GERD, sinusitis, and pneumonia were reported more often in severe asthma. These results support smaller studies that have used similar methods of self-report to estimate prevalence of comorbid diseases in severe asthma.19,34–37 In our study, nearly 2/3 of the severe group reported a history of pneumonia and, except for baseline FEV1 % predicted, pneumonia was the strongest independent predictor of severe asthma (OR, 3.30). Recurrent respiratory infections requiring antibiotics have been shown to be a risk factor (OR, 6.9) for severe exacerbations.37 Asthma has recently been reported to be a risk factor for invasive pneumococcal disease, and this risk increased with increasing disease severity.38 These data suggest that pneumonia may be a contributing factor in the development of severe asthma and that respiratory infections may lead to further morbidity in patients with severe asthma. The increased prevalence of pneumonia in severe asthma requires further investigation into potential alterations in innate immunity mechanisms that may be common in these 2 diseases.
A previously described subphenotype of severe asthma based on age of asthma onset (early vs late) was evaluated in the SARP cohort.9,10 Early-onset severe asthma was distinguished by more allergic responses (skin tests, specific allergic symptoms), longer disease duration, and higher lifetime HCU. In contrast, late-onset asthma was characterized by a reduced FVC and a history of more frequent sinopulmonary infections, perhaps related to low baseline lung function. These data suggest that different immunologic mechanisms may be responsible for the development of severe asthma in early-onset compared with late-onset severe asthma.
There are some limitations to this study. First, although the majority of the data represent objective physiologic parameters, subjective or historical data were assessed by using questionnaires that may be subject to recall bias. Questionnaires were standardized among sites and administered by clinic staff rather than self-administered, because self-administration has been reported to underestimate disease.39 The recall period of the different SARP questionnaires ranged from current events to those in the past 3 months (medications and symptoms) to lifetime recall (medical history and HCU) with the caveat that 95% of our subjects were younger than 43 years. Questionnaires were designed to assess asthma characteristics in the severe asthma cohort with milder disease as a reference group. Cross-reference of daily albuterol use with reported daily symptoms showed excellent concordance in 3/4 of the subjects with severe asthma, suggesting that questionnaire data are accurate in this group. Although recall bias may be possible in the milder asthma groups, we stress that these groups were the reference group for the severe group and that the frequencies of symptoms and HCU in the severe group are the data that should be emphasized.
Another limitation of this study concerns the definitions used to classify asthma severity (both severe and not severe). The majority of our subjects, particularly the subjects with severe asthma, were recruited from the subspecialty clinics of SARP investigators. Although estimates of patient compliance with ICS vary widely in the literature, several reports have noted increased medication adherence with increasing severity of asthma and greater compliance in patients prescribed ICS/LABA combinations.40–43 As has become standard of care (even if not endorsed by guidelines), the majority (75%) of our subjects on ICS reported use of ICS/LABA combinations, and most reported worsening of asthma with reduction in corticosteroid dose, suggesting that the importance of adherence to prescribed asthma therapies is recognized by these subjects. We did not specifically measure patient adherence as part of this study and cannot exclude noncompliance as a confounder in disease classification, especially in the not severe asthma groups, which were not rigorously defined a priori. Although some of the not severe subjects may have been undertreated, the incremental worsening in measured outcomes from mild to severe disease found in SARP suggests that the classification scheme used in this study is appropriate. In fact, if the subjects with not severe asthma could have been improved with better therapy, this would only have increased the disparity between severe and milder asthma groups.
In conclusion, this study confirms that severe asthma is characterized by persistent asthma symptoms, abnormal lung function that is very responsive to the acute administration of bronchodilators, increased medication use, and significant comorbidities that result in disproportionate utilization of health care resources. A reduced FEV1 (although not specific for severe asthma), a history of pneumonia, and fewer skin test responses were the strongest independent risk factors for severe asthma, but a significantly higher incidence of sinus disease and GERD was also observed. Although the evaluation of the SARP cohort supports the differentiation between early-onset and late-onset severe asthma, it is likely that other subphenotypes will be identified through the ongoing mechanistic studies being performed in SARP.
Acknowledgments
Supported by HL69116, HL69130, HL69149, HL69155, HL69167, HL69170, HL69174, HL69349, M01 RR018390, M01 RR007122-14, and M01 RR03186.
Abbreviations used
- ATS
American Thoracic Society
- ED
Emergency department
- FeNO
Exhaled nitric oxide
- FVC
Forced vital capacity
- GERD
Gastroesophageal reflux disease
- GINA
Global Initiative for Asthma
- HCU
Health care utilization
- ICS
Inhaled corticosteroid
- ICU
Intensive care unit
- LABA
Long-acting β-agonist
- NHLBI
National Heart, Lung, and Blood Institute
- OCS
Oral corticosteroid
- OR
Odds ratio
- SABA
Short-acting β-agonist
- SARP
Severe Asthma Research Program
Footnotes
Disclosure of potential conflict of interest: S. C. Erzurum has received grant support from Alair. L. Bacharier is on the speakers’ bureau for AstraZeneca, GlaxoSmithKline, Genentech, and Merck. W. J. Calhoun has consulting arrangements with Critical Therapeutics and Genentech. B. D. Levy has consulting arrangements with Critical Therapeutics. W. G. Teague is on the speakers’ bureau for Merck. W. W. Busse has consulting arrangements with Genentech/Novartis, Isis, GlaxoSmithKline, Altana, Wyeth, Pfizer, Dynavax, and Centocor, has received grant support from Novartis, Wyeth, Dynavax, Centocor, and GlaxoSmithKline, and is on the speakers’ bureau for GlaxoSmithKline, Novartis, Merck, and Astra-Zeneca. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–1326. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 2.Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilization. Eur Respir J. 2004;23:723–729. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 3.Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity. Eur Respir J. 2002;19:61–67. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000;106:1033–1042. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel SE. Severe asthma in adults. Am J Respir Crit Care Med. 2005;172:149–160. doi: 10.1164/rccm.200409-1181PP. [DOI] [PubMed] [Google Scholar]
- 6.Dolan CM, Fraher KE, Bleecker ER, Borish L, Chipps B, Hayden ML, et al. for the TENOR Study Group. Design and baseline characteristics of the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study-a large cohort of patients with severe or difficult-to-treat-asthma. Ann Allergy Asthma Immunol. 2004;92:32–39. doi: 10.1016/S1081-1206(10)61707-3. [DOI] [PubMed] [Google Scholar]
- 7.Miller MK, Johnson C, Miller DP, Deniz Y, Bleecker ER, Wenzel SE for the TENOR Study Group. Severity assessment in asthma: an evolving concept. J Allergy Clin Immunol. 2005;116:990–995. doi: 10.1016/j.jaci.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins HA, Cherniack R, Szefler SJ, Covar R, Gelfand EW, Spahn JD. A comparison of the clinical characteristics of children and adults with severe asthma. Chest. 2003;124:1318–1324. doi: 10.1378/chest.124.4.1318. [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age of onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Bumbacea D, Campbell D, Nguyen L, Carr D, Barnes PJ, Robinson D, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24:122–128. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health, National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert Panel report 2: guidelines for the diagnosis and management of asthma. Bethesda (MD): National Institutes of Health; 1997 #97-4051.
- 13.National Institutes of Health, National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert Panel report: guidelines for the diagnosis and management of asthma: update on selected topics 2002. Bethesda (MD): National Institutes of Health; 2002 #02-5075.
- 14.National Institutes of Health, National Heart, Lung, and Blood Institute. Global Initiative for Asthma. Bethesda (MD): National Institutes of Health; 2002:1–176. #02-3659.
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society and European Respiratory Society. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 17.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 18.Colice GL. Categorizing asthma severity: an overview of national guidelines. Clin Med Res. 2004;2:155–163. doi: 10.3121/cmr.2.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicenter study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 20.Diette GB, Krishnan JA, Wolfenden LL, Skinner EA, Steinwachs DM, Wu AW. Relationship of physician estimate of underlying asthma severity to asthma outcomes. Ann Allergy Asthma Immunol. 2004;93:546–552. doi: 10.1016/S1081-1206(10)61261-6. [DOI] [PubMed] [Google Scholar]
- 21.Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. Am J Respir Crit Care Med. 1998;157:1804–1809. doi: 10.1164/ajrccm.157.6.9708092. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell I, Tough SC, Semple LK, Green FH, Hessl PA. Near-fatal asthma: a population-based study of risk factors. Chest. 2002;121:1407–1413. doi: 10.1378/chest.121.5.1407. [DOI] [PubMed] [Google Scholar]
- 23.Griswald SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA. Asthma exacerbations in North American adults: who are the “frequent fliers” in the emergency department? Chest. 2005;127:1579–1586. doi: 10.1378/chest.127.5.1579. [DOI] [PubMed] [Google Scholar]
- 24.Eisner MD, Boland M, Tolstykh I, Mendoza G, Iribarren C. Intensive care unit admission for asthma: a marker for severe disease. J Asthma. 2005;42:315–323. doi: 10.1081/JAS-62959. [DOI] [PubMed] [Google Scholar]
- 25.Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 26.Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol. 1996;98:522–527. doi: 10.1016/s0091-6749(96)70085-4. [DOI] [PubMed] [Google Scholar]
- 27.Ricciardolo F, Sterk P, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lim S, Jatakanon A, Meah S, Oates T, Chung KF, Barnes PJ. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax. 2000;55:184–188. doi: 10.1136/thorax.55.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–1255. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax. 1998;53:1030–1034. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweik R, Comhair S, Gaston B, Thunnissen F, Farver C, Thomassen M, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates CA, Silkoff PE. Exhaled nitric oxide in asthma: from bench to bedside. J Allergy Clin Immunol. 2003;111:256–262. doi: 10.1067/mai.2003.103. [DOI] [PubMed] [Google Scholar]
- 33.Comhair SAA, Ricci KS, Arroliga M, Lara AR, Dweik RA, Song W, et al. for the NHLBI Severe Asthma Research Program. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172:306–313. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koga T, Oshita Y, Kamimura T, Koga H, Aizawa H. Characterization of patients with frequent exacerbation of asthma. Respir Med. 2006;100:273–278. doi: 10.1016/j.rmed.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Liou A, Grubb JR, Schechtman KB, Hamilos DL. Causative and contributive factors to asthma severity and patterns of medication use in patients seeking specialized asthma care. Chest. 2003;124:1781–1788. doi: 10.1378/chest.124.5.1781. [DOI] [PubMed] [Google Scholar]
- 36.Bresciani M, Paradis L, Des Roches A, Vernhet H, Vachier I, Godard P, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 37.ten Brinke A, Sterk PJ, Masclee AAM, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factor of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 38.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann MM, Jacobs EJ, Hoffmann K, Boeing H. Agreement of self-reported medical history: comparison of an in-person interview with a self-administered questionnaire. Eur J Epidemiol. 2004;19:411–416. doi: 10.1023/b:ejep.0000027350.85974.47. [DOI] [PubMed] [Google Scholar]
- 40.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices and inhalation techniques. Chest. 2000;117:542–550. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 41.Diette GB, Wu AW, Skinner EA, Markson L, Clark RD, McDonald RC, et al. Treatment patterns among adult patients with asthma. Arch Intern Med. 1999;159:2697–2704. doi: 10.1001/archinte.159.22.2697. [DOI] [PubMed] [Google Scholar]
- 42.De Smet BD, Erickson SR, Kirking DM. Self-reported adherence in patients with asthma. Ann Pharmacother. 2006;40:414–420. doi: 10.1345/aph.1G475. [DOI] [PubMed] [Google Scholar]
- 43.Stoloff SW, Stempel DA, Meyer J, Stanford RH, Rosenzweig RC. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–251. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]