Abstract
IL-4 induces a lipase, pancreatic lipase related protein 2 (PLRP2), in cytotoxic T lymphocytes (CTLs). Because PLRP2 in semen can mediate lipid-dependent toxicity to sperm, we questioned whether CTL-derived PLRP2 could support similar cytotoxicity towards tumor cells. Recombinant PLRP2 was toxic to P815 tumor cells in 48 hours when lipid and another protein, colipase, were present. However, PLRP2-positive CTLs (induced with many lots of IL-4) were unable to mediate lipid-dependent cytotoxicity. Notably, CTLs induced with only one lot of IL-4 had lipid-dependent cytotoxicity. The exceptional lot of IL-4 was effective in multiple experiments at inducing lipid-dependent cytotoxicity. The lipid-dependent cytotoxicity it induced was determined to be perforin-independent. CTLs induced with IL-4 that was unable to induce lipid-dependent cytotoxicity had mRNA for PLRP2 but not mRNA for colipase. Therefore, we added exogenous colipase to the CTL assays but still cytotoxicity was unchanged. We conclude (1) that lipid-dependent cytotoxicity, promoted by the lipase PLRP2 and colipase, will kill tumor cells and (2) that more than PLRP2 alone is required for lipid-dependent cytotoxicity mediated by CTLs.
Keywords: IL-4, lipase, PLRP2, linoleic acid, cytotoxic T lymphocytes
INTRODUCTION
Lipases are recognized enzymes of the pancreas with extensively characterized roles in the digestion of dietary fats 1,2. However, in other sites of the body, lipases may have other functions. In this paper we consider if a lymphocyte lipase has a role in lipid-dependent CTL-mediated cytotoxicity. Several immune cells express lipases 3,4,5,6, leading us to query what the lipase roles might be. Macrophages produce and secrete lipoprotein lipase 7,8,9,10 and IL-4 induced T lymphocytes (T cells) produce a lipase, called pancreatic lipase related protein 2 (PLRP2) 3,4,5. Lymphocytes grown in the presence of triglycerides hydrolyzed the lipid to release fatty acids, providing further evidence for a T cell-derived lipase 11.
Support for lipases with immunological function came from studies of PLRP2−/− cytotoxic T lymphocytes (CTLs) 5. PLRP2−/− T cells taken ex vivo displayed reduced cytotoxicity compared to T cells taken from wild type littermates. In addition to a potential direct role in T cell-mediated cytotoxicity, PLRP2 exhibited indirect toxic effects on sperm samples 12,13. In these studies, PLRP2 was identified as the enzyme responsible for the rapid reduction in the viability of goat semen during preparation for cryopreservation. The loss of sperm viability was caused by fatty acids released by PLRP2 from milk triglycerides that were added to the buffers used for cryopreservation.
Unsaturated fatty acids such as arachidonic, as well as linoleic and oleic acids at elevated concentrations can cause apoptotic as well as necrotic death of tumor cells 14,15,16,17,18,19. Tumor cells grown in unsaturated fatty acids have increased caspase activity and DNA fragmentation14,15,16,20. We investigated if the IL-4 induced T cell lipase, PLRP2, or another similar lipase found within cytotoxic T lymphocytes, may mediate indirect killing by release of fatty acids from triglycerides.
Lymphatic and serum fluids contain triglycerides in the form of chylomicrons and very low density lipoproteins (VLDLs) and thus the lipids are available to serve as substrates to support lipase-dependent cytotoxicity. Triglycerides are customarily viewed as energy storage molecules found within the body and are circulated through both the lymphatic and vascular systems. Each triglyceride is composed of a glycerol backbone with three fatty acid chains attached. During the breakdown of triglycerides by extracellular lipases, fatty acid chains are released from the glycerol, bound to albumin, and transported into cells that oxidize the fatty acid chains to produce ATP. As part of critical cellular nutrition, triglycerides are ubiquitously present in tissue and organs where they could support additional functions.
The objectives of the study were to see if pure PLRP2 lipase could be cytotoxic and if PLRP2-positive CTLs would demonstrate similar activity. We used two approaches, one with r-PLRP2 and the other with CTLs. Using recombinant human PLRP2 (rPLRP2), we first asked if the lipase could indirectly mediate death of P815 tumor cells in the presence of triglyceride lipids. PLRP2 with triglyceride lipids did mediate indirect toxicity to P8195 cells while direct treatment of tumor cells with rPLRP2 without triglycerides was ineffective at inducing death. We next investigated how similar triglyceride conditions would affect cytotoxicity mediated by IL-4 induced, PLRP2-positive CTLs. Long term viability assays of P815 cells attacked by CTLs in the presence of triglycerides were used to allow time for accumulation of enzymatically generated fatty acids. The cytotoxic activity of PLRP2-positive CTLs was unchanged by the addition of triglycerides, except for CTLs induced with one particular lot of IL-4. These CTLs had increased cytotoxicity in the presence of lipids. The lipid-dependent cytotoxicity was consistent for the one lot, though non-reproducible with other lots of IL-4. Attempts were made to reproduce the lipid dependent toxicity by adding other variables, including blockade of type 1-polarizing cytokines in culture (which might counteract IL-4 type 2 T cell induction) and addition of the lipase cofactor, colipase (which increases PLRP2 activity) to the cytotoxicity assays.
In summary, we have found that purified PLRP2 together with triglyceride lipid and colipase can mediate indirect cytotoxicity. We also found that PLRP2+ CTLs induced with IL-4 alone lack triglyceride-enhanced cytotoxicity. In addition, we acquired evidence for triglyceride-enhanced CTL-mediated cytotoxicity.
METHODS AND MATERIALS
Materials
Recombinant (r-) mouse IL-2 was purchased from eBioscience (San Diego, CA, with specific activity of 1×107 units/mg; 1.7×1011 units/mmole). r-Mouse IL-4 was purchased from BD Biosciences (San Jose, CA, 2.5×107 units/mg, 3.5×1011 units/mmole) or from eBioscience (San Diego, CA, 1×107 units/mg, 1.4×1011 units/mmole). For the lot numbers see tables 1 and 2. Anti-mouse IL-12 (eBioscience).
Table 1.
Triglyceride-enhanced cytotoxicity of IL-4 induced CTLs
| IL-4 lotb | Mouse strainsc | Lytic units per 106 CTLs | Lipid enhancementf | |
|---|---|---|---|---|
| IL-4 CTLsd | IL-4 CTLs + lipide | |||
| BD: 60506 | Balb/c WT | 17902 | 29574 | 1.7 |
| BD: 60506 | Balb/c WT | 20837 | 29250 | 1.4 |
| BD: 60506 | Balb/c WT | 17125 | 23825 | 1.4 |
| BD: 60506 | Balb/c perforin KO | 24041 | 74989 | 3.1 |
| BD: 60506 | Balb/c perforin KO | 10955 | 38416 | 3.5 |
| BD: 60506 | Balb/c perforin KO | 2359 | 11936 | 5.1 |
| BD:83653 | Balb/c WT | 11915 | 9402 | 0.8 |
| BD:83653 | Balb/c WT | 22222 | 25763 | 1.2 |
| BD:83653 | Balb/c perforin KO | 28529 | 29749 | 1.0 |
| BD:83653 | Balb/c perforin KO | 23378 | 27227 | 1.2 |
| eBio:E028417 | Balb/c WT | 9824 | 8734 | 0.9 |
| eBio:E028417 | Balb/c perforin KO | 23820 | 23433 | 1.0 |
| eBio:E031047 | Balb/c WT | 35815 | 21322 | 0.6 |
| eBio:E031047 | Balb/c perforin KO | 30388 | 37775 | 1.2 |
| eBio:E031047 | C57B6 | 35815 | 21322 | 0.6 |
| eBio:E031047 | C57B6 perforin KO | 15226 | 11749 | 0.8 |
Cells were cultured for 5 days with 500 units/ml of different lots of IL-4.
The vendors and lots of IL-4. BD, BD Biosciences; eBio, eBioscience.
Mouse strains and genetic modifications.
Lytic units per 10M CTLs for IL-4 induced CTLs without triglycerides.
Lytic units per 10M CTLs for IL-4 induced CTLs with triglycerides.
Ratios of the lytic activity of CTLs assayed in the presence of lipid compared to CTLs without lipid.
Table 2.
Lipid-dependent cytotoxicity following alternative methods of CTL induction
| IL-4 lota | Conditionsb | Activationc | Mouse straind | Lytic units per 106CTLs | Lipid enhancementg | |
|---|---|---|---|---|---|---|
| IL-4 CTLse | IL-4 CTLs + lipidf | |||||
| BD:83653 | 1500 U/ml IL-4 | ConA | Balb/c WT | 16031 | 16950 | 1.1 |
| eBio:E031047 | 1500 U/ml IL-4 | ConA | Balb/c perforin KO | 23246 | 31979 | 1.4 |
| eBio:E031047 | 500 U/ml IL-4 | ConA | IFNg receptor KO B6 | 26246 | 16407 | 0.6 |
| eBio:E031047 | 500 U/ml IL-4 + anti-IL-12 | ConA | IFNg receptor KO B6 | 46398 | 16408 | 0.4 |
| eBio:E031047 | 500 U/ml IL-4 | Anti-CD3/CD28 | C57B6 | 30063 | 27780 | 0.9 |
| eBio:E031047 | 500 U/ml IL-4 | Anti-CD3/CD28 | C57B6 | 23088 | 27931 | 1.2 |
| eBio:E031047 | 500U/ml IL-4 + 400U/ml IFNg | ConA | C57B6 | 3543 | 1351 | 0.4 |
Lot of IL-4.
Concentrations of cytokine during induction of T cells.
Method of T cell activation.
Mouse strain and genetic modifications.
Lytic units per 10M CTLs for IL-4 induced CTLs assayed without triglycerides.
Lytic units per 10M CTLs for IL-4 induced CTLs assayed with triglycerides.
Ratios of the lytic activity of CTLs assayed in the presence of lipid compared to CTLs without lipid.
Animal Studies
The animal protocols for this study were approved by the University of Nevada, Reno Institutional Animal Care and Use Committee.
Cell Culture
Splenocytes were harvested from the following strains of mice: Balb/c mice wild-type (Jackson Labs, Bar Harbor, ME), BALB/c perforin−/− mice 21 (kindly provided by Dr Thomas Sayers, the National Cancer Institute (NCI, Fredrick, MD)), C57BL/6 IFN-γ receptor−/− (provided by Dr. William J. Murphy, University of Nevada, Reno) and C57BL/6 mice (purchased from the NIH-NCI referral service). Cells were cultured at 6.5×105 cells/ml in T -75 flasks (Starstedt) with complete tissue culture media containing RPMI-1640 (Sigma Chemical Co, St. Louis, MO), 10% fetal calf serum (HyClone, Logan, UT), 10 mM HEPES buffer (Fisher Scientific, Waltham, MA), 1% Pen-Strep (Sigma), 24 mM sodium bicarbonate (Fisher), 25 μM 2-mercaptethanol (Sigma), 2.5 μg/ml concanavalin A (conA) (Sigma), and 500 units per ml of either IL-2 or IL-4 to induce the splenocytes. Following 3 days of culture with conA and cytokines, the T cells were washed to remove the mitogen conA and re-cultured in complete tissue culture medium lacking conA with either IL-4 or IL-2 for an additional 2 days. In experiments where plate bound anti-CD3 and anti-CD28 were substituted for conA stimulation, 10 μg/ml anti-CD3 and 20 μg/ml anti-CD28 were used to initially coat plates. eGFP-P815 mastocytoma cells 22 were maintained in Dulbecco’s Modified Eagle Medium, DMEM, (Gibco, Grand Island, New York) with 18 mM sodium bicarbonate (Fisher), 1 μg/ml puromycin (Sigma) and 10% FBS (HyClone). All cell cultures were incubated at 37°C and 5% CO2.
Measurement of lipase activity released by CTLs
Lipase activity released by CTLs was determined by measuring the 3H-fatty acid released from 3H-triolein during anti-CD3 induced exocytosis. To create media with lipid micelles, 0.312 mM unlabeled triolein (Sigma) was added to complete RPMI-1640 media followed by the addition of 1.14×10−7 mM 3H-triolein (spec. act. 91 Ci/mmol, Perkin Elmer, Waltham, Massachusetts). The lipid containing media was vortexed for 3 minutes to create micelles. The 135 μls of micelle-containing media were plated out into each well of a 96 well plate. Following the micelles, 135 μls of media containing CTLs and anti-CD3 (clone 4-2C11) (eBioscience) coated beads was added to each well. The assay was incubated at 37°C with 5% humidity for 4 hours. Following this incubation, 200 μls cells and media were collected and the released fatty acids were extracted using 800 μls of a chloroform/methanol/heptanes solution (14.5 parts/12 parts/10 parts). The resulting extraction was made basic with the addition of 250 μls from a 50 mM carbonate (Sigma) solution, pH 10. The phase separation of the fatty acids and lipid layers was created by centrifugation at 2600 rpm (1000 g) for 5 minutes at room temperature. From the top phase containing fatty acids, 200 μls was collected, and mixed with 500 μls of scintillation cocktail, (MicroScint-20, Perkin Elmer) and counted in a Beckman scintillation counter (LS 6000IC Fullerton, CA).
51Cr redirected lysis assays
IL-2 or IL-4 induced CTLs cytotoxicity was tested using 8 hour redirected lysis assays with eGFP-P815 mastocytoma cells as radio-labeled targets. eGFP-P815 target cells were labeled with Na51CrO4 (Perkin Elmer) 23 for 1 hour. T cells were redirected to the IgG Fc receptor-bearing P815 cells with 1 μg/ml anti-mouse CD3 mAb (clone 4-2C11) (eBioscience, San Diego, CA). For control conditions, T cells were incubated with 51Cr labeled P815s in the presence of golden Syrian hamster IgG (14-4914-85, eBioscience, San Diego, CA). Assays were run in quadruplicate with 10,000 radio-labeled cells per well for 8 hours with and without 1.5 mM trilinolein (Sigma). Trilinolein (Sigma) was dissolved in ethanol, vortexed in media and added to the CTL: P815 mixtures. The final ethanol concentration in all assays did not exceed 0.07%, which we found to lack detectable toxicity toward CTL or P815s (data not shown).
Specific release was calculated by the formula:
Cytometric tumor outgrowth assays
On day 5, CTLs were plated out with eGFP-P815s at specified effector to target ratios in the presence of 1 μg/ml anti-CD3 (clone 4-2C11) (eBioscience) or with 1 μg/ml of golden Syrian hamster IgG (eBioscience, San Diego, CA). Trilinolein (Sigma) was dissolved in ethanol, vortexed in media, and added to the CTL: P815 mixtures as above. The assay was incubated at 37°C with 5% CO2 for 48 hours. Upon completion of the incubation, cells were harvested and measured with a Beckman Coulter XL/MCL flow cytometer. Live and dead eGFP-P815s were distinguished from one another based on GFP fluorescence and forward scatter properties. To avoid counting errors caused by cell doublets and debris from dead cells, fluorescent counting beads (Polymer Labs, Amherst, MA) were added to each sample and used to normalize the viable P815 cell number within each sample. The data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). In some experiments, JURKAT cells (ATCC) were evaluated as alternate targets, monitoring live cells by forward and side light scatter properties.
Lipase and fatty acid toxicity towards tumor cells
Tumor cell viable recovery after 48 hours was monitored by flow cytometry with eGFP-P815 cells or JURKAT cells as above, using purified lipases with or without cofactor instead of CTLs. The P815s lost green fluorescence and the FL1 bright cell population was depleted when cells died. The JURKAT cells appeared as a high forward scatter cell population, which was depleted when the JURKAT cells died; the decomposing cells appeared as a separate lower forward scatter population. Purified recombinant human PLRP2 (kindly provided by Frederick Carriere, Ph.D.), porcine pancreatic triglyceride lipase (PTL) (Sigma, 47,900 units/mg) and porcine colipase (AbD Serotec) (4,210 units/mg) were used alone or as combinations of lipase and colipase. The lipid in these assays was 1.5 mM Trilinolein added as above. In other experiments, linoleic acid (Sigma) was dissolved in ethanol and added to the eGFP-P815s for 2 days.
Lipid product toxicity assay
Day 5 IL-4 induced T cells were harvested and plated out with anti-CD3 (clone 4-2C11) (eBioscience) coated streptavidin beads (Polysciences, Inc., Warrington, PA) in the presence of 1.5 mM trilinolein. The assay was incubated at 37°C with 5% CO2 for 48 hours. Upon completion of the incubation, cells and media were collected and spun in 1.5 ml Eppendorf tubes at 10,000 rpm (10,200 g) for 10 minutes. The cell-free supernatant was collected and was added 1:1 to media (+/− fresh trilinolein) containing eGFP-P815s at a concentration of 200,000 cells per ml. The assays were incubated at 37°C with 5% CO2 for another 48 hours. Viable P815 cells were measured by flow cytometry as described above.
Quantitative RT-PCR
Taqman® quantitative RT-PCR was performed with TaqMan® FAM-dye labeled probes for PLRP2, colipase, and cyclophilin mRNAs and for 18s ribosomal RNA per the manufacturer’s directions. Cyclophilin and 18s RNA were used as internal calibration controls to adjust for differences in the amounts of mRNA in different samples. Purified pancreatic total RNA (Biochain) was used as a positive control for colipase expression.
Statistics and reproducibility
Results are expressed as mean +/− standard error of the mean of the collected data. Statistical evaluations were performed with Excel and Sigma plot software applying Student’s T-test. Differences were considered significant if the P value was <0.05. Unless otherwise indicated in the figure legends, the experiments illustrated are representative of three or more similar experiments.
RESULTS
PLRP2, together with lipid substrates, mediates indirect toxicity toward tumor cells
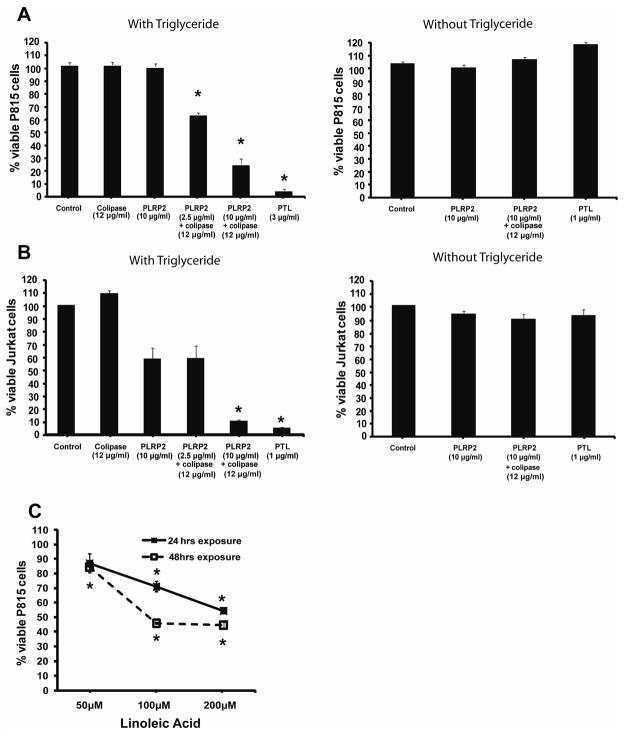
We questioned if the lipase, PLRP2, was directly or indirectly toxic towards tumor cells. We incubated recombinant PLRP2 with P815 cells, with or without triglyceride lipids in the presence or absence of the lipase cofactor, colipase, over a 48 hour period. Native purified pancreatic triglyceride lipase (PTL) was used as a control because it is a similar enzyme and is commercially available. Both PLRP2 and PTL were unable to damage P815 cells directly (Figure 1A). With the lipases and their cofactor, colipase, one might expect direct toxicity to be enhanced; however, direct toxicity was still absent (illustrated for PLRP2 in Figure 1A). To monitor indirect toxicity (from lipid products), we added lipid substrate to the cellular assays together with the lipases. P815 viability was reduced when PLRP2 was combined with the triglyceride, trilinolein, and colipase (Figure 1A).
Figure 1. PLRP2 with triglyceride lipid and colipase mediated indirect toxicity toward tumor cells.
A and B eGFP-P815 cells (A) or JURKAT cells (B) were incubated with 2.5 or 10 μg/ml rPLRP2 and 50 units/ml (12 μg/ml) colipase, with and without trilinolein, for 48 hours. The fluorescent viable P815s and the intact JURKATS (determined by forward and side scatter properties) were counted by flow cytometry. The error bars in this figure and the following figures represent standard errors of the mean. The asterisks indicate P values <0.05 for reduction of viable cells compared to control cells that were cultured without lipases. rPLRP2 together with colipase significantly reduced tumor cell recovery when triglycerides were present. PTL is another pancreatic lipase (that is unexpressed by lymphocytes) and has greater enzyme activity towards triolein under these conditions. C. eGFP-P815 cells were incubated with varying concentrations of linoleic acid for 48 hours. P815 cells died at 100 micromolar and greater concentrations of linoleic acid.
JURKAT cells were also tested as tumor cell targets for direct and indirect cytotoxicity by PLRP2 or by PTL. JURKAT cells were similarly invulnerable to direct attack (in the absence of exogenous lipids) and susceptible to indirect toxic effects by either lipase plus cofactor when combined with lipid substrate (Figure 1B). PLRP2 alone (without cofactor but with triglyceride lipid) at 10 μg/ml mediated modest indirect cytotoxicity toward JURKAT cells (Figure 1B), which suggests that JURKAT cells are more sensitive than P815 cells.
We established that fatty acid products from the lipid substrates are potential toxic mediators. These lipases will release 2 or 3 molecules of linoleic acid from each molecule of trilinolein. The toxicity generated by each lipase in the presence of triglycerides was similar to the toxicity observed when P815 cells were incubated with high concentrations of linoleic acid for 48 hours (Figure 1C). The 1.5 mM trilinolein substrate in the assays and the activities of the lipases would be sufficient to produce the 100+ micromolar concentrations of fatty acids needed. Furthermore, addition of delipidated bovine serum albumin (BSA) at physiological concentrations blocked the toxic effects (not illustrated). The BSA found in serum can bind as much as 3.6 mM fatty acid (calculated at a buffering capacity of 7 molecules of fatty acid per molecule of albumin 24).
Triglyceride-hydrolyzing lipases are secreted by IL-4-induced CTLs
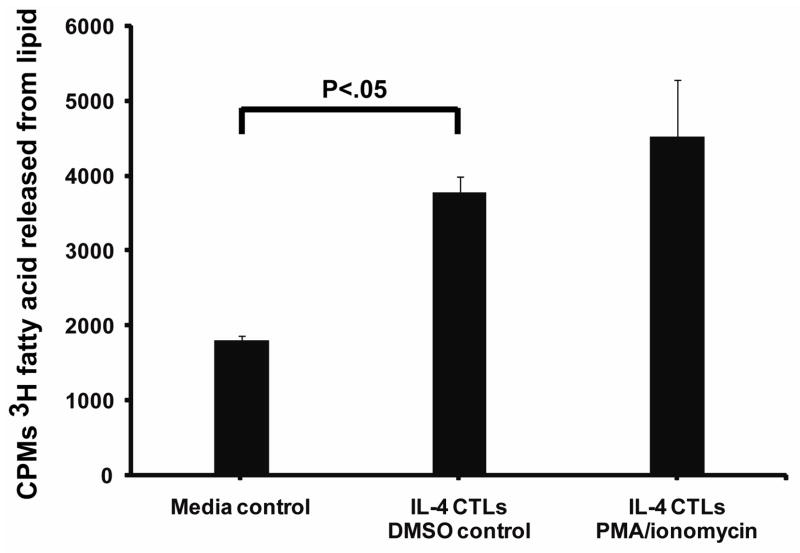
The potential methods of PLRP2 (or other lipases) release from CTLs include constitutive secretion, a burst upon antigen receptor-stimulated exocytosis and/or prolonged secretion after the CTLs have encountered their antigens. To simulate antigen recognition, we treated the CTLs with either phorbol myristate acetate (PMA) combined with an ionophore (ionomycin) 25 or with anti-CD3ε covalently bound to beads 26, a signaling protein associated with the T cell receptor for antigen. IL-4 induced T cells were stimulated with PMA and ionomycin or with DMSO as a solvent control for 4 hours in media with 3H-triolein as a reactive substrate that was incorporated into lipid micelles. The cell-free supernatant was collected and tested for the release of radio-labeled fatty acids from 3H-triolein. Supernatants from DMSO-treated CTLs displayed triglyceride lipase activity that was above background, indicating that there was some constitutive secretion of lipase (Figure 2). Fetal calf serum in the media contributed lipase activity to the background (Figure 2). This background hydrolysis of 3H-triolein handicapped detection of the cellular lipases; however, it was necessary to retain fetal calf serum in these experiments in order to reduce the death of T cells. The CTL-mediated constitutive secretion of lipase compared to the media had a P<0.05 in two of three experiments, including the one illustrated. Therefore the CTLs constitutively secrete lipase activity. Stimulation with PMA and ionomycin or anti-CD3 (data not illustrated) failed to significantly increase lipase release over constitutive secretion in two of three experiments (Figure 2).
Figure 2. CTLs secreted triglyceride lipase(s).
CTLs cultured for 5 days with 500 u/ml (1.4 × 10−9 M) lot 83653 IL-4 were incubated for 4 hours with lipid micelles that contained 3H-triolein as a substrate to detect lipase activity. PMA and ionomycin were used to initiate degranulation and cytokine release from the CTLs. The fatty acids released by lipases into the cell-free supernatants were separated from the lipid by extraction with organic solvents. The lipase present in the media control is due to the addition of fetal calf serum.
We gained additional information from these assays. Calibration with rPLRP2 as an internal standard indicated that there would be less than 30 ng equivalents of PLRP2 secreted per 106 IL-4 CTLs, assuming that all the secreted lipase activity was mediated by only PLRP2. CTLs grown in IL-2 also had constitutively secreted triglyceride lipase(s) but there was no evidence of stimulated release of lipase (data not illustrated).
IL-4 cultured CTLs lacked lipid-dependent cytotoxicity; however, CTLs induced by one exceptional lot of IL-4 had lipid-dependent cytotoxicity
To determine if the addition of the triglyceride, trilinolein, to CTL cytotoxicity assays would support lipid-dependent cytotoxicity, we performed two types of cytotoxic killing assays. One assay depended on 8 hour 51Cr-release and was substantially perforin dependent while the other assay utilized fluorescent P815 cells in a 48 hour tumor viability assay. CTLs from wild type (perforin-positive) mice induced with IL-4 mediated similar 8 hour cytotoxicity with or without lipid, regardless of the lot of IL-4 (data not illustrated). To favor detection of lipid-dependent cytotoxicity which could be covered up by perforin activity, we used CTLs from perforin deficient (perforin−/−) mice.
With perforin−/− CTLs without lipid, there were low levels of cytotoxicity at 4 hours, but moderate levels at 8 hours. All lots of IL-4 tested induced expression of PLRP2 mRNA in CTLs detectable by Q RT-PCR and by immunoblots (Alves et al., submitted). Despite the presence of PLRP2, cytotoxicity was not enhanced by the inclusion of lipid (Figure 3A). In fact, cytotoxicity was sometimes suppressed by the lipid, as indicated in Figure 3A. CTLs induced with IL-2 were also unable to mediate lipid-dependent cytotoxicity (data not illustrated).
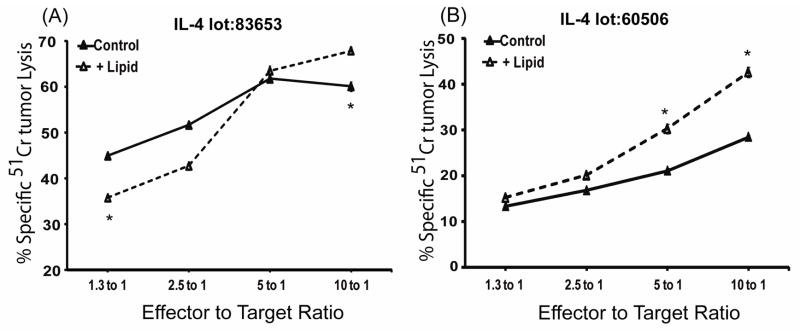
Figure 3. CTLs mediated lipid-dependent cytotoxicity in 8 hour 51Cr-release assays only when they were induced with an exceptional lot of IL-4.
Perforin−/− CTLs, cultured with different lots of IL-4 at 500 u/ml, were incubated with 51Cr-radio-labeled P815 cells in anti-CD3 redirected assays that were stopped after 8 hours. The CTL assays were performed with or without trilinolein. The standard errors of the means are illustrated but are so small that they often appear to merge with the symbols. (A). CTLs cultured with lot 83653 IL-4 lacked lipid-enhanced cytotoxicity. This lack of lipid dependent activity was standard and reproducible with multiple lots of IL-4. (B). CTLs cultured with lot 60506 IL-4 displayed lipid-enhanced cytotoxicity by 8 hours. This figure illustrates data representative of two experiments with this lot of IL-4.
However, perforin−/− CTLs, when induced with an exceptional lot of IL-4 (BD lot 60506), demonstrated lipid-enhanced cytotoxicity (Figure 3B). CTLs with this exceptional lot had lipid enhanced cytotoxicity at 8 hours (Figure 3B) but not 4 hours (not shown). Since perforin-dependent cytotoxicity is readily observed within 4 hours, the data indicate that lipid-dependent cytotoxicity is much slower than perforin-dependent cytotoxicity.
Thus we obtained a clear indication of lipid-enhanced cytotoxicity with CTLs induced with one exceptional lot of IL-4 (which contained IL-4 and probably an additional factor). We continued our investigations with lot 60506 IL-4, without knowledge at the time that the lot was exceptional.
Flow cytotometry assays for long term lipid-dependent cytotoxicity, monitored by viable tumor cell recovery
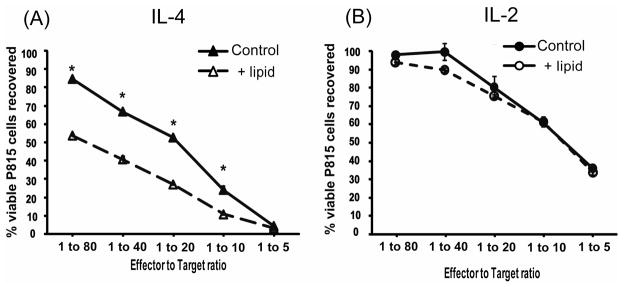
Longer term assays, in which apoptotic killing mediated by WT and perforin−/− CTLs is equally effective (without lipid), were performed next. Trilinolein was added to 48 hour anti-CD3 redirected lysis of fluorescent eGFP-P815 cells using IL-2 and IL-4 induced CTLs. For reference, the viability of P815 cells was unaffected when the P815s were incubated with lipid alone. With most lots of IL-4, cytotoxicity was the same with or without lipid, mediated either by WT or perforin−/− CTLs. (See Table 1 and a following section in which additional variables were added to stimulate lipid-dependent killing.)
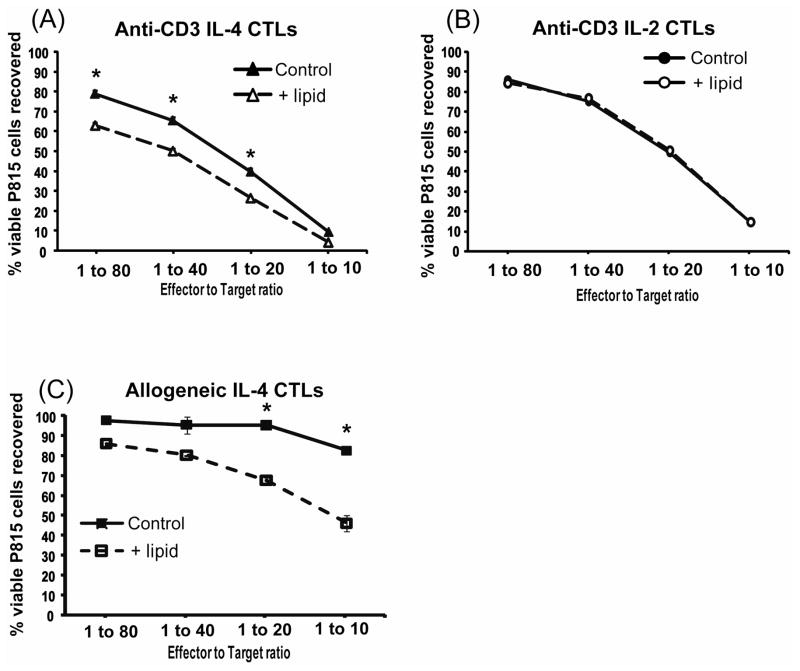
With the exceptional lot of IL-4 (and only this lot of IL-4), viable P815 cells were reduced in the triglyceride-containing assays when compared to the assays without lipids (Figure 4A). Thus CTLs do have the potential to mediate lipid-dependent cytotoxicity. This lipid-dependent cytotoxicity was reproduced 3 times with WT CTLs and three times with perforin−/− CTLs (Table 1). The difference between lipid-dependent and lipid-independent cytotoxicity was slight but statistically significant for the WT cells. The triglyceride-enhanced cytotoxicity was absent in the long term killing assays using IL-2 induced WT CTLs (Figure 4B).
Figure 4. CTLs mediated lipid-dependent cytotoxicity in long term tumor viability assays only when they were induced with an exceptional lot of IL-4.
The effects of trilinolein addition to IL-2 and lot 60506 IL-4 induced wild type CTLs were tested in anti-CD3 redirected tumor viability assays in (A) and (B). These assays used CTLs incubated with eGFP-P815s for 48 hours and were assessed by flow cytometry as in Figure 1. A. Lot 60506 IL-4 induced CTLs mediated lipid-dependent cytotoxicity. The ratio of lipid-dependent to lipid-independent cytotoxicity for this experiment, determined by lytic units, was 1.7. B. IL-2 induced CTLs showed no enhancement with the addition of triglycerides. The CTLs from the same mouse as in A were induced with 500 u/ml IL-2. (C). Allogeneic long term tumor viability assays were used to evaluate if the lipid-enhanced killing would occur without anti-CD3 redirected CTL engagement of the tumor cells. The lot 60506 IL-4-induced CTLs displayed triglyceride lipid-dependent cytotoxicity. The ratio of lipid-dependent to lipid-independent cytotoxicity for this experiment, determined by lytic units, was six (10459 LU per 10M CTLs/1737 LU per 10M CTLs.
CTL induced with the exceptional lot of IL-4 were also tested for lipid-dependent cytotoxicity in allogeneic killing assays in which the anti-CD3 antibody was omitted (Figure 4C). The BALB/c effectors and the P815 cells are both H-2d but differ at minor histocompatibility loci27. In the allogeneic system, these IL-4 induced cells displayed higher cytotoxic activity toward P815s when lipid was present (Figure 4C). In summary, CTLs induced with an exceptional lot of IL-4 acquired a novel lipid-dependent method for mediating tumor cell death.
The long term triglyceride-enhanced cytotoxicity by the CTLs induced with the exceptional lot of IL-4 was perforin-independent
Perforin is a primary mediator of the cytotoxic activity utilized by CTLs. Perforin deficient CTLs have dramatically less cytotoxic capacity than their wild type counterparts in short term assays 28. We questioned if the triglyceride-enhanced cytotoxic activity was perforin dependent or independent using the long term killing assays with IL-2 or IL-4 induced CTLs from perforin−/− mice. Perforin−/− CTLs induced with the exceptional lot of IL-4 had enhanced cytotoxicity upon the addition of triglyceride to the long term assays (Figure 5A). Perforin−/− IL-2 induced CTLs showed no enhancement upon the addition of triglycerides (Figure 5B). The triglyceride-enhanced cytotoxicity was easier to detect with the perforin−/−CTLs than with the WT CTLs (compare Figure 5A with Figure 4A). The lipid-enhanced cytotoxicity was consistent in 3 experiments with perforin−/− CTLs, using BD lot 60506 IL-4 (Table 1).
Figure 5. Triglyceride enhanced CTL activity was perforin-independent.
IL-4 (A) and IL-2 (B) induced CTLs were generated from a perforin knockout Balb/c mouse to determine if the triglyceride enhancement was perforin-independent. Triglyceride enhancement was observed after the CTLs were induced with lot 60506 IL-4. The ratio of lipid-dependent to lipid-independent cytotoxicity for this experiment, determined by lytic units, was 3.1. The lipid-dependent cytotoxicity was absent from Perforin−/− IL-2 induced CTLs, as well as the WT IL-2 CTLs illustrated in Figure 4.
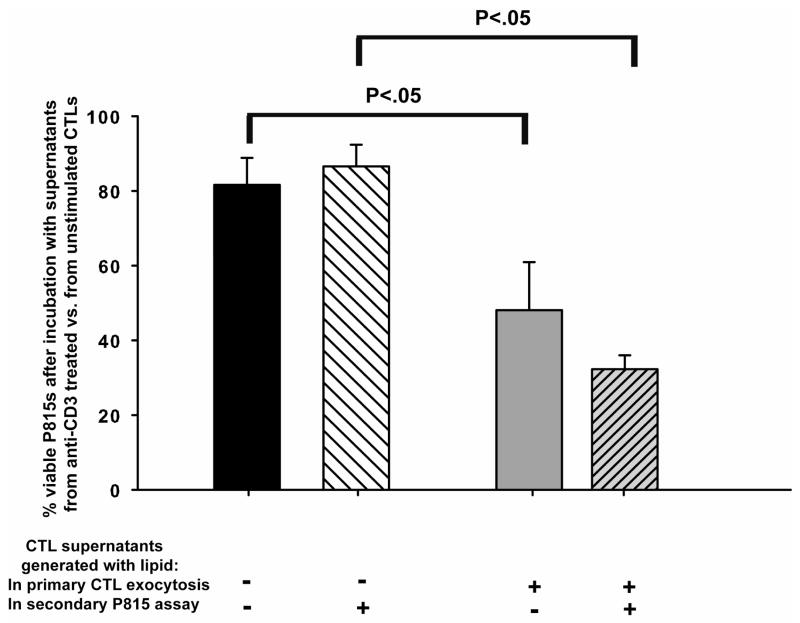
The CTLs induced with the exceptional lot of IL-4 generated toxic products in the presence of lipid
To determine if the lipid-enhanced killing was mediated by toxic products produced from the lipid, CTLs were stimulated to undergo exocytosis in the presence or absence of triglycerides and cell-free supernatants were evaluated for toxicity. IL-4 induced CTLs were used because they had PLRP2 lipase expression (which might produce fatty acids to contribute to cytotoxicity). The IL-4 induced CTLs were generated from perforin−/− mice because anti-CD3 triggers the release of toxic concentrations of perforin that will kill some of the CTLs even though released perforin is very unstable. Exocytosis from these perforin−/− IL-4 induced CTLs was stimulated with anti-CD3 covalently coated beads for 48 hours. Following exocytosis, cell-free supernatants were collected and added to eGFP-P815 cells and the cells plus supernatants were incubated for 48 hours. Lipid was present under three conditions: (1) only during the initial 48 hours of CTL exocytosis (to test for lipase activity upon the lipid); or (2) the lipid was present only during the second 48 hours of incubation of the CTL supernatants with eGFP-P815s (to test for a released lipase that might remain active in the cell-free supernatants and later be able to convert lipids into toxic products). As a last condition (3), lipid was present during both the 48 hours of CTL exocytosis and during the incubation of the supernatants with the P815s. We found that P815 cell viability was reduced by the cell-free supernatants that were collected from CTLs undergoing exocytosis in the presence of triglycerides (Figure 6, bars 3 and 4, numbering 1–4 from left to right). Bar 3 illustrates the loss of viability when lipid was present during only the initial CTL anti-CD3 stimulation. Bar 4 illustrates the loss of viability when lipid was present during both the CTL anti-CD3 stimulation and the incubation of the P815s with the cell-free supernatants.
Figure 6. CTLs induced with lot 60506 IL-4 and stimulated with anti-CD3 antibodies produced soluble toxic products in the presence of triglyceride lipids.
These experiments had 2 steps. In step 1, supernatants were generated after stimulation with anti-CD3 or without stimulation and collected in the presence or absence of triglyceride lipids. In step 2, these supernatants were tested for release of either lipases or lipid byproducts that might be toxic when incubated with P815 cells. In each step, lipid was a variable, to test for the presence of lipases. CTLs induced with lot 60506 IL-4 were stimulated to undergo exocytosis using anti-CD3 coated beads for 48 hours in the presence or absence of triglycerides. Control supernatants were collected from CTLs under matched culture conditions that contained beads without anti-CD3. Following the initial 48 hour stimulated exocytosis, the CTL cell-free supernatants were collected and added to eGFP-P815 cells. The P815 cells were in media with or without fresh triglycerides in the hope of detecting released lipases and were incubated for 48 hours with the cell-free supernatants. The controls for this experiment that determined 100% viability were the supernatants from the un-stimulated CTLs (beads minus anti-CD3). These supernatants were without effect on P815 growth and survival (not illustrated). The cell-free supernatants generated in the presence of triglycerides (bars 3 & 4 numbered 1–4 from the left) were toxic compared to the cell-free supernatants produced without triglycerides during exocytosis. The lipid was essential as in its absence in during primary exocytosis (bars 1 & 2) there was little cytotoxicity elicited by anti-CD3 stimulation. Furthermore, these experiments indicate that anti-CD3 stimulation was essential to produce soluble toxins in the presence of lipids.
The loss of viability was consistent with lipase and lipid-dependent cytotoxicity. When lipid was missing during CTL exocytosis, there was little subsequent effect on P815 viability (Figure 6, bar 1). Inclusion of lipid in the P815 assays with the CTL-derived supernatants generated without lipid had no effect. The controls that represented 100% tumor cell viability for each bar in figure 6, were P815 cells exposed to supernatants from CTLs incubated without anti-CD3 antibody (with or without lipid). These supernatants of un-stimulated cells lacked cytotoxicity. Thus, we could generate toxic supernatants only from anti-CD3 treated CTLs and only when lipid was present during the initial anti-CD3 stimulation. We interpret this toxic activity as evidence that a released lipase may be able to generate toxic products from lipid.
We consumed all of lot 60506 IL-4 before we realized its unique qualities. The other lots of IL-4 did not support the production of soluble toxic products. These findings concerning the CTLs and their cell-free supernatants indicate that lipid-enhanced killing can be generated by CTLs.
Efforts to stimulate triglyceride-enhanced cytotoxicity
The triglyceride-enhanced cytotoxicity was missing when other lots of IL-4 were used to induce these cells (see Table 1). Initially the potency of the additional lots was suspected to be lower than BD lot 60506 and to be unable to induce PLRP2. Multiple lots of IL-4, from different vendors, induced mRNA for PLPR2 (data not illustrated).
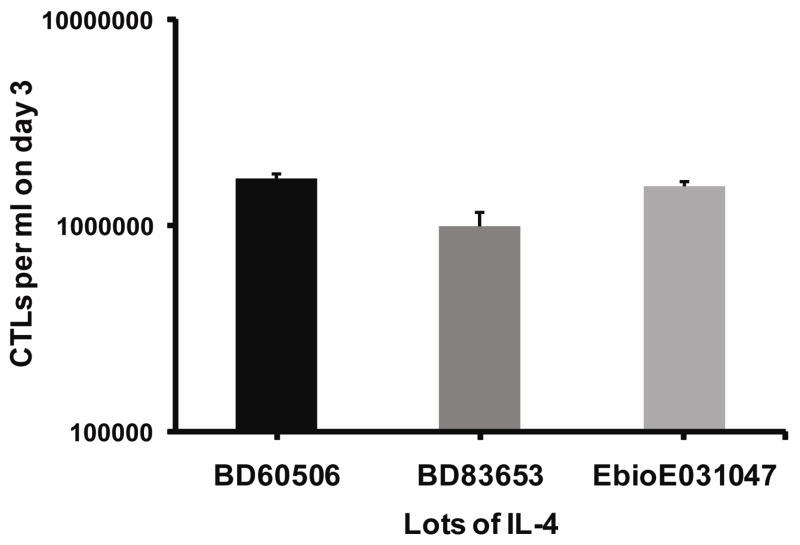
IL-4 is a growth factor for T cells, and, if the ineffective lots of IL-4 were less potent, then T cell growth would be reduced. However, the T cells growth rates from day 0 to day 3 were similar with each lot of IL-4, suggesting a separate factor was responsible for the triglyceride-enhanced killing (Figure 7). The growth rates from day 3 (when the conA was removed) to day 5 were similar (not illustrated). IL-4 at the 500 units/ml in the assays with the various lots varied from 1.4to 3.5 ×10−9 M and the IL-4 receptors of T cells (comprised of the IL-4 alpha receptor chain with the common gamma cytokine receptor chain) have a Kd of 1.1×10−10 M, indicating that the receptors were saturated by each lot of IL-4. These results suggest that the critical variable was something other than IL-4 and also that this variable was effective in the presence of active IL-4.
Figure 7. The different lots of IL-4 had similar bioactivity as reflected by growth of CTLs.
Cells were cultured at 650,000 cells per ml with different lots of IL-4 at 500 units/ml. The viable cells recovered were counted by Trypan blue exclusion on day 3.
Attempts were also made to reproduce the triglyceride-enhanced toxicity by altering several variables (Table 2). Increasing the IL-4 concentration (of the ineffective lots of IL-4) was without effect. We questioned if lipopolysaccharide was responsible for the triglyceride-enhanced cytotoxicity, but reports from the vendors indicated that the effective lot 60506 had less LPS than the ineffective lots of IL-4. Following these results, we questioned whether lack of the triglyceride-enhanced toxicity could be caused by variation in type 1 responses in the CTL cultures. Both IL-12 and IFN-γ are the dominant cytokines associated with the generation of a type 1 response in naive T cells 29,30. To reduce the type 1 polarizing effects of IFN-γ and IL-12, IFN-γ receptor−/− splenocytes were induced with an ineffective lot of IL-4 in the presence or absence of 20 μg/ml IL-12 blocking antibodies. Again, these conditions were unsuccessful at reproducing the triglyceride enhanced killing. We also tested for effects of type 1 polarization by adding high concentrations of mouse IFN-γ during splenocyte activation with IL-4. However, these experiments were performed with ineffective lots of IL-4 and, as might be expected, the triglyceride-enhanced killing was still absent (Table 2). We tested if stimuli other than conA could activate triglyceride-enhanced cytotoxicity. We used plate bound anti-CD3/CD28 to activate splenocytes instead of conA activation. This induction method was also unsuccessful at reproducing the lipid-enhanced toxicity originally observed in the IL-4 induced CTLs (Table 2).
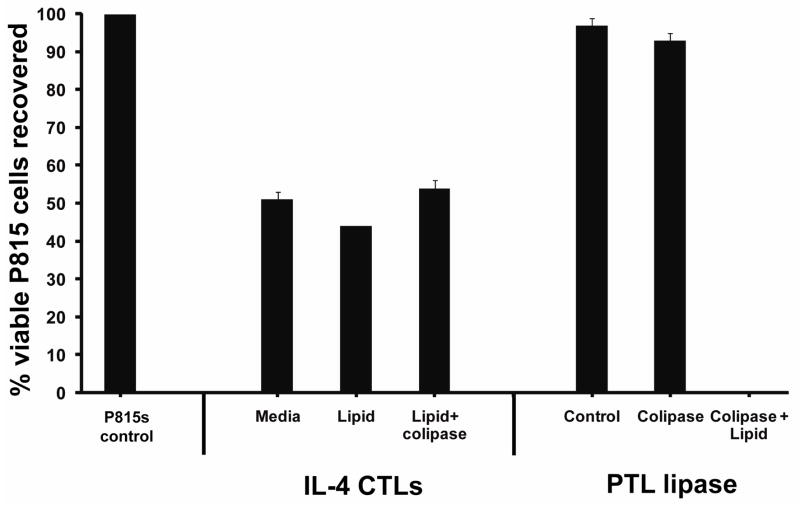
Another possible explanation for the triglyceride-dependent killing would be the coincident CTL expression of the cofactor that increases the activity of pancreatic lipases, a small protein termed colipase. Colipase increases both PTL and PLRP2 activity toward triglycerides 31,32,33. We tested for the presence of colipase in lymphocytes by Taqman® RT-PCR, using pancreatic mRNA for calibration, and were unable to detect any mRNA for colipase in lymphocytes. Based on the detection threshold, the relative amount of colipase mRNA in CTLs was less than 1 × 10−6 that of the pancreas.
However, a search of the NCBI’s GEO Profiles Gene Expression Omnibus database indicated that under certain conditions T lymphocytes will express colipase. (See Geo Profiles: GDS993, GDS2649 and GDS1336.) We wondered if an unknown factor associated with the exception lot 60506 of Il-4 could have induced colipase. Since lot 60506 was consumed, direct information was impossible. As an alternative approach, we tested if the addition of purified colipase to CTLs induced with standard lots of IL-4 would support lipid-enhanced cytotoxicity. The colipase increased the triglyceride-enhanced cytotoxicity mediated by purified PTL (used as a control to indicate the efficacy of the colipase) but the colipase was without effect on the IL-4 induced CTLs. At this time, we can confidently report that PLRP2-positive CTLs induced with standard lots of IL-4 are unable to mediate the triglyceride-dependent cytotoxicity that was observed for recombinant PLRP2 with colipase and P815 cells above. In addition, the results associated with IL-4 lot 60506 indicate the existence of CTL-mediated triglyceride lipid-dependent cytotoxicity.
DISCUSSION
Here we discuss and provide perspectives on three aspects of our research on lipid-dependent CTL-mediated cytotoxicity. We are the first to report that a lipase expressed by CTLs will generate products in the presence of triglyceride lipids that are toxic to tumor cells. Next, in the second aspect of the research, we examine the ability of CTLs to support triglyceride-dependent killing of tumor cells, and discovered that CTLs expressing the same lipase, PLRP2, are normally unable to kill by a lipid-dependent mechanism. We present highly reproducible results with many lots of IL-4 to support this latter conclusion. As a third aspect of this report, we venture to present additional findings that are consistent with one exceptional lot of IL-4 but that are non-reproducible with other lots of IL-4. It is important to report these experiments because they indicate that there is potential for CTLs to acquire this unique mechanism. At the end of the discussion, we recommend future approaches that could guide researchers willing to pursue a novel lipid-dependent CTL mechanism.
The most positive results of our research are the discovery that lipases can cause toxicity to tumor cells. Two lipases, PTL, a digestive lipase of the pancreas, and PLRP2, found in the pancreas, IL-4 induced CD8 cytotoxic lymphocytes (Alves et al., manuscript submitted), and testes, were toxic to tumor cells if appropriate triglyceride lipid substrates were provided. Without lipids, the lipases were nontoxic, indicating that toxicity was indirect and mediated by triglyceride byproducts. Two tumor lines, murine P815s that are somewhat resistant to apoptosis and the apoptosis-sensitive human JURKAT cells, were killed when incubated in the presence of lipase and triglyceride lipids. The products generated from the lipid, trilinolein, during lipase hydrolysis include the fatty acid linoleic acid, di- and mono-glycerides. Research considering the effects of fatty acids on tumor cells has demonstrated that, at high concentrations, unsaturated fatty acids are toxic to different tumor cells 14,16,34,35. Normally most fatty acids are either bound to albumin or are covalently attached to glycerol to form triglycerides. Lipases, such as those used here, are employed by the body to release fatty acids from triglycerides. Our experiments using linoleic acid revealed toxicity toward tumor cells can be caused by 48 hour exposure to concentrations of 100 μM linoleic acid and above. The death caused by linoleic acid was indistinguishable by flow cytometry from lipid-dependent death caused by CTLs. The lipid concentration used in our studies combined with the enzymatic activity of these lipases would be sufficient to generate fatty acids in excess of toxic concentrations of 100 μM within hours. This information was derived from measurement of the enzymatic activities of the two lipases in the tissue culture media with radioactive triolein as a tracer in the lipid micelles to measure fatty acid production (not illustrated). Furthermore, albumin was used as probe for the biochemical nature of the soluble toxins. Assays carried out in media containing physiological (35 mg/ml) concentrations of albumin (which binds 7 fatty acid molecules per molecule of albumin) blocked the toxicity of the purified lipases incubated with P815s, suggesting that released fatty acids are responsible for the observed toxicity (data not shown).
Next we consider the cell biology of lipid-dependent cytotoxicity and the likelihood that it might occur in vivo. P815 cell death from fatty acids requires more than 24 hours. This requirement corresponds to the time required for the death observed for tumor cells incubated with the combination of lipase, lipid, and cofactor and for the lipid-dependent CTL-mediated cytotoxicity. Tumor cell death in vivo can also take more than 24 hours. In vivo efficacy would require sufficient lipid substrate, which does occur since millimolar concentrations of triglyceride lipids are found in serum incorporated into very light density lipoproteins (VLDLs) and present in chylomicrons 36. Considerations for practical tumor therapy include potentially neutralizing conditions, such as the physiological clearance of the toxic products via the circulation of blood and lymph and the buffering of fatty acids by serum albumin. Both of these mechanisms could reduce fatty acids to nontoxic levels. However, for perspective, tumor micro-environments may have regions with low circulation and low serum albumin, where CTLs could be invasive effector cells.
We observed a lipid-dependent form of CTL cytotoxicity when redirected lysis was carried out in the presence of lipids over a 48 hour period that was readily detectable in the absence of perforin. These results reveal a previously unknown mechanism through which CTLs may kill tumor cells. In addition to having found that triglycerides could enhance the cytotoxic effects of T cells, we determined that the induced T cells could generate transferable toxic productions in the presence of triglycerides. It is reasonable to infer that the toxins were derived from lipid; however, biochemical proof will require discovery of the conditions needed to induce this toxicity and measurement of lipid products in the cell supernatants with toxic activity.
Even though multiple lots of IL-4 other than lot 60506 invariably induced PLRP2 in CTLs (manuscript submitted), these IL-4 induced CTLs lacked lipid-dependent cytotoxicity. Still, the results from the exceptional lot of IL-4 indicate that CTLs can mediate lipid-dependent cytotoxicity. Although a new mechanism of cytotoxicity is possible, it may be prudent to view this phenomenon as mere evidence that the CTLs can secrete lipase(s). Furthermore, this potent lipase (or lipases) may actually be intended biologically for cellular nutrition instead of as a cytotoxic mechanism 11.
We close this discussion with thoughts for the future. The method of preparation of the exceptional lot of IL-4, BD 60506, was expression in insect cells infected with IL-4 encoding baculovirus followed by isolation of the IL-4 by antibody affinity chromatography. Many contaminants (other than LPS) could have been present, including viral products, antibody-IL-4 immune complexes, and even bacterial products in buffers. Unfortunately, the vendor was unable to provide more of lot 60506 for characterization. The potential contaminants are able to stimulate Toll receptors or FcγR receptors associated with antigen-antibody complexes and suggest that stimulation of these receptors would be worth investigating. Viral nucleic acids or other viral components stimulate Toll receptors 2 and 9 37,38,39. Immune complexes stimulate both macrophage and dendritic cells through the FcγR receptor 40,41. Toll receptors are found on dendritic cells and macrophages, both of which were present in our splenocyte cultures. Stimulation via different Toll receptors or other receptors alters dendritic cells and macrophages and may lead to critical changes in communication between T cells and dendritic cells during differentiation of the T cells into activated CTLs. We recommend that future investigations focus on stimulation of different Toll receptors. We also recommend that future studies consider the use of trilinolein as the lipid substrate, long term tumor viability assays to monitor lipid enhanced toxicity, and use of perforin−/− CTLs from BALB/c mice which are a ‘type 2’-responder strain 42. It is important to address this new lipid-dependent cytotoxicity because of its potential to contribute to both cancer immunity and to pathology.
Figure 8. Purified colipase increased lipid-dependent cytotoxicity mediated by PTL but was without effect on the cytotoxic activity CTLs induced with a standard lot of IL-4.
The CTLs were induced for 5 days with lot E031047 IL-4 and expressed PLRP2 mRNA (not illustrated). CTLs or the lipase PTL were combined with or without colipase and tested in 48 hour tumor viability assays in the presence or absence of trilinolein. Tumor viability was unchanged by the addition of colipase to CTLs incubated in the presence of lipid when compared to assays lacking lipids. In assays containing PTL, tumor viability was significantly reduced upon addition of colipase when lipid was present.
Acknowledgments
The authors thank William J. Murphy, Ph.D. (UC Davis), and Jennifer V. Welser, Ph.D. (Scripps Research Institute) for their critical comments, and Frederick Carriere, Ph.D. (CNRS UPR9025 Laboratoire d’Enzymologie Interfaciale et de Physiologie de la Lipolyse, Marseille, France) for the generous gift of r-PLRP2. This study was supported in part by NIH R01CA38942-20 (DH), R01HD33060 and DK053100 (M.L.) and T32 09563 (B.N.A. and D.T.), a University of Nevada Undergraduate Fellowship (J.L.), a GSA graduate student grant and the NIH Nevada INBRE P20RR016463 for flow cytometry.
Abbreviations
- CD
cluster of differentiation
- ConA
concanavalin A
- CTLs
cytotoxic T lymphocytes
- E:T
effector lymphocyte to tumor target ratio
- Grz
granzyme
- Ig
immunoglobulin
- IL
interleukin
- eGFP-P815
a mastocytoma tumor cell line which expresses green fluorescent protein used as a target
- PLRP2
pancreatic lipase-related protein 2
- PMA
phorbol myristic acid
- PTL
pancreatic triglyceride lipase
- r-
recombinant
- VLDL
very low density lipoproteins
References
- 1.Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 3.Grusby MJ, Nabavi N, Wong H, Dick RF, Bluestone JA, Schotz MC, Glimcher LH. Cloning of an interleukin-4 inducible gene from cytotoxic T lymphocytes and its identification as a lipase. Cell. 1990;60:451–459. doi: 10.1016/0092-8674(90)90596-7. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MH, Boyer SN, Grusby MJ. Genomic organization of the murine CTL lipase gene. Genomics. 1996;35:606–609. doi: 10.1006/geno.1996.0407. [DOI] [PubMed] [Google Scholar]
- 5.Lowe ME, Kaplan MH, Jackson-Grusby L, D’Agostino D, Grusby MJ. Decreased neonatal dietary fat absorption and T cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J Biol Chem. 1998;273:31215–31221. doi: 10.1074/jbc.273.47.31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe ME. Properties and function of pancreatic lipase related protein 2. Biochimie. 2000;82:997–1004. doi: 10.1016/s0300-9084(00)01184-6. [DOI] [PubMed] [Google Scholar]
- 7.Chait A, Iverius PH, Brunzell JD. Lipoprotein lipase secretion by human monocyte-derived macrophages. J Clin Invest. 1982;69:490–493. doi: 10.1172/JCI110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoo JC, Mahoney EM, Witztum JL. Secretion of lipoprotein lipase by macrophages in culture. J Biol Chem. 1981;256:7105–7108. [PubMed] [Google Scholar]
- 9.Mahoney EM, Khoo JC, Steinberg D. Lipoprotein lipase secretion by human monocytes and rabbit alveolar macrophages in culture. Proc Natl Acad Sci U S A. 1982;79:1639–1642. doi: 10.1073/pnas.79.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoo JC, Vance JE, Mahoney EM, Jensen D, Wancewicz E, Steinberg D. Neutral triglyceride lipase in macrophages. Arteriosclerosis. 1984;4:34–40. doi: 10.1161/01.atv.4.1.34. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC, Yaqoob P, Newsholme EA. Triacylglycerol metabolism by lymphocytes and the effect of triacylglycerols on lymphocyte proliferation. Biochem J. 1994;298:605–611. doi: 10.1042/bj2980605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellicer-Rubio MT, Magallon T, Combarnous Y. Deterioration of goat sperm viability in milk extenders is due to a bulbourethral 60-kilodalton glycoprotein with triglyceride lipase activity. Biol Reprod. 1997;57:1023–1031. doi: 10.1095/biolreprod57.5.1023. [DOI] [PubMed] [Google Scholar]
- 13.Pellicer-Rubio MT, Combarnous Y. Deterioration of goat spermatozoa in skimmed milk-based extenders as a result of oleic acid released by the bulbourethral lipase BUSgp60. J Reprod Fertil. 1998;112:95–105. doi: 10.1530/jrf.0.1120095. [DOI] [PubMed] [Google Scholar]
- 14.Cury-Boaventura MF, Pompeia C, Curi R. Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin Nutr. 2004;23:721–732. doi: 10.1016/j.clnu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Cury-Boaventura MF, Curi R. Regulation of reactive oxygen species (ROS) production by C18 fatty acids in Jurkat and Raji cells. Clin Sci (Lond) 2005;108:245–253. doi: 10.1042/CS20040281. [DOI] [PubMed] [Google Scholar]
- 16.Cury-Boaventura MF, Pompeia C, Curi R. Comparative toxicity of oleic acid and linoleic acid on Raji cells. Nutrition. 2005;21:395–405. doi: 10.1016/j.nut.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Finstad HS, Myhrstad MC, Heimli H, Lomo J, Blomhoff HK, Kolset SO, Drevon CA. Multiplication and death-type of leukemia cell lines exposed to very long-chain polyunsaturated fatty acids. Leukemia. 1998;12:921–929. doi: 10.1038/sj.leu.2401030. [DOI] [PubMed] [Google Scholar]
- 18.Finstad HS, Heimli H, Kolset SO, Drevon CA. Proliferation and types of killing of leukemia cell lines by very long chain polyunsaturated fatty acids. Lipids. 1999;34 (Suppl):S107. doi: 10.1007/BF02562250. [DOI] [PubMed] [Google Scholar]
- 19.Heimli H, Finstad HS, Drevon CA. Necrosis and apoptosis in lymphoma cell lines exposed to eicosapentaenoic acid and antioxidants. Lipids. 2001;36:613–621. doi: 10.1007/s11745-001-0765-x. [DOI] [PubMed] [Google Scholar]
- 20.Lima TM, Kanunfre CC, Pompeia C, Verlengia R, Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol In Vitro. 2002;16:741–747. doi: 10.1016/s0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 21.Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 22.Kienzle N, Olver S, Buttigieg K, Kelso A. The fluorolysis assay, a highly sensitive method for measuring the cytolytic activity of T cells at very low numbers. J Immunol Methods. 2002;267:99–108. doi: 10.1016/s0022-1759(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 23.Bevan MJ, Langman RE, Cohn M. H-2 antigen-specific cytotoxic T cells induced by concanavalin A: estimation of their relative frequency. Eur J Immunol. 1976;6:150–156. doi: 10.1002/eji.1830060303. [DOI] [PubMed] [Google Scholar]
- 24.Huber AH, Kampf JP, Kwan T, Zhu B, Kleinfeld AM. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45:14263–14274. doi: 10.1021/bi060703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama H, Sitkovsky MV. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J Exp Med. 1987;166:725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancki DW, Kaper BP, Fitch FW. The requirements for triggering of lysis by cytolytic T lymphocyte clones. II. Cyclosporin A inhibits TCR-mediated exocytosis by only selectively inhibits TCR-mediated lytic activity by cloned CTL. J Immunol. 1989;142:416–424. [PubMed] [Google Scholar]
- 27.Korngold R, Doherty PC. The localized primary cytotoxic T-cell response to cells expressing minor histocompatibility differences. Scand J Immunol. 1984;19:175–180. doi: 10.1111/j.1365-3083.1984.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 28.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–431. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 31.De Caro J, Sias B, Grandval P, Ferrato F, Halimi H, Carriere F, De Caro A. Characterization of pancreatic lipase-related protein 2 isolated from human pancreatic juice. Biochim Biophys Acta. 2004;1701:89–99. doi: 10.1016/j.bbapap.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Eydoux C, De Caro J, Ferrato F, Boullanger P, Lafont D, Laugier R, Carriere F, De Caro A. Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells. J Lipid Res. 2007;48:1539–1549. doi: 10.1194/jlr.M600486-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Jennens ML, Lowe ME. Rat GP-3 is a pancreatic lipase with kinetic properties that differ from colipase-dependent pancreatic lipase. J Lipid Res. 1995;36:2374–2382. [PubMed] [Google Scholar]
- 34.Andrade LN, de Lima TM, Curi R, Castrucci AM. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol In Vitro. 2005;19:553–560. doi: 10.1016/j.tiv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Anel A, Naval J, Desportes P, Gonzalez B, Uriel J, Pineiro A. Increased cytotoxicity of polyunsaturated fatty acids on human tumoral B and T-cell lines compared with normal lymphocytes. Leukemia. 1992;6:680–688. [PubMed] [Google Scholar]
- 36.Vance DE, Vance JE. Biochemistry of lipids, Lipoproteins and Membranes. 5. 2008. pp. 485–490. [Google Scholar]
- 37.Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 40.Ronnelid J, Ahlin E, Nilsson B, Nilsson-Ekdahl K, Mathsson L. Immune complex-mediated cytokine production is regulated by classical complement activation both in vivo and in vitro. Adv Exp Med Biol. 2008;632:187–201. [PubMed] [Google Scholar]
- 41.Bjorck P, Beilhack A, Herman EI, Negrin RS, Engleman EG. Plasmacytoid dendritic cells take up opsonized antigen leading to CD4+ and CD8+ T cell activation in vivo. J Immunol. 2008;181:3811–3817. doi: 10.4049/jimmunol.181.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]