Abstract
We are developing a Salmonella vectored vaccine to prevent infant pneumonia and other diseases caused by Streptococcus pneumoniae. One prerequisite for achieving this goal is to construct and evaluate new recombinant attenuated Salmonella vaccine (RASV) strains suitable for use in neonates and infants. Salmonella enterica serovar Typhimurium strain χ9558(pYA4088) specifies delivery of the pneumococcal protective antigen PspA and can protect adult mice from challenge with S. pneumoniae. This strain is completely safe for oral delivery to day-old and infant mice. Here we assess the colonizing ability, immunogenicity, and protective efficacy of χ9558(pYA4088) in neonatal mice. Colonization was assessed in mice 0, 2, 4, or 7 days of age after oral inoculation. In the presence of maternal antibodies, the colonization of lymphoid tissues was delayed, but the immune responses were enhanced in mice born to immunized mothers. Both oral and intranasal routes were used to assess immunogenicity. All orally or intranasally immunized neonatal and infant mice born to either immunized or naïve mothers developed PspA-specific mucosal and systemic immune responses. Mice born to immunized mothers produced higher titers of PspA-specific antibodies in the blood and mucosa and greater numbers of PspA-specific interleukin-4 (IL-4)-secreting cells than mice born to naïve mothers. More importantly, mice born to immune mothers showed a significant increase in protection against S. pneumoniae challenge. These results suggest that strain χ9558(pYA4088) can circumvent some of the limitations of the immature immune system in neonatal and infant mice, generating enhanced protective immune responses in the presence of maternal antibodies.
Streptococcus pneumoniae is a respiratory pathogen that enters the body through the respiratory mucosa (45) and may cause diseases such as pneumonia, otitis media, meningitis, and bacteremia, especially in young children and the elderly (24). Moreover, it is a leading cause of pneumonia in young children, resulting in an estimated 1 to 3 million deaths each year (16, 40). An increase in the incidence of antibiotic-resistant S. pneumoniae is a growing problem worldwide (1, 10), and infants are colonized at an early age in countries where resistant strains are prevalent (31). Fortunately, the use of antipneumococcal vaccines can prevent antibiotic-resistant infections and limit the development of drug resistance. A 7-valent pneumococcal conjugate vaccine (Prevnar) was licensed in 2000 by Wyeth and has been used for children under the age of 2 years (5). Although this vaccine has proven useful, capsular types not covered by the vaccine have emerged (18, 44), leaving young children once again vulnerable to S. pneumoniae infection and disease. Prevnar 13, which includes five additional serotypes, is currently under review by the FDA (34).
In our laboratory, we have been developing a vaccine for the prevention of S. pneumoniae infections based on surface protein antigens, such as PspA and PspC (7, 9). Our strategy has been to use live attenuated Salmonella vectors to deliver the relevant antigens (23, 27, 33, 48, 49). One challenge of early-life immunization arises as a consequence of the limited immune responses in neonates and infants (43). Successful induction of a protective response must circumvent the typically weak and short-lived antibody response of the immature immune system and the inhibitory influence of maternal antibodies (42). In a previous study, a live attenuated Salmonella vaccine was used to induce a strong immune response in the face of an immature immune system and maternal antibodies (11).
While safety and immunogenicity are the two most important factors to consider in developing a live recombinant attenuated Salmonella vaccine (RASV), when the vaccine is targeted toward infants and young children, safety becomes paramount. We have recently reported the development of several new strategies to enhance both RASV safety and immunogenicity, including regulated delayed attenuation (12, 13, 27), regulated delayed antigen synthesis (49), programmed cell lysis (25), and a constellation of other mutations, such as ΔsopB, which enhances the immune response against a vectored antigen (28, 29) and reduces fluid accumulation in the intestines (16a, 50). Salmonella enterica serovar Typhimurium strain χ9558 (16a) has many of these new features.
We have taken a balanced approach to our strain construction strategy, adding features to improve both immunogenicity and safety. As a result, strain χ9558 has demonstrated an improved safety profile in adult mice, with a reduced ability to cause meningitis when administered orally, intranasally (i.n.), or intraperitoneally (i.p.) (6), and it is totally safe and noninflammatory in newborn mice at doses equal to 107 times the 50% lethal dose (LD50) of the wild-type parent (16a). Plasmid pYA4088 is an Asd+ balanced-lethal plasmid that carries the gene for an immunogenic portion of the protective PspA antigen fused to a type 2 secretion signal for β-lactamase, directing secretion of the fusion protein to the periplasm and outside the cell (21, 23, 49). When χ9558 carrying a plasmid nearly identical to pYA4088 was used to immunize adult mice, the mice were significantly protected against challenge with 200 times the LD50 of virulent S. pneumoniae (27). The high level of protection was comparable to the protection observed in mice immunized with an RASV lacking many of these new-generation vaccine safety features and was significantly greater than the protection afforded by a Δcya Δcrp RASV lacking any of the new-generation features.
In this work we confirmed the safety of χ9558(pYA4088) for young mice and examined the immunogenicity and protective efficacy of χ9558(pYA4088) for neonatal and infant mice born to naïve and immunized mothers. In a previous study, Capozzo et al. demonstrated both the safety and the immunogenicity of a live attenuated Salmonella strain when it was administered by the intranasal route (11). Our ultimate goal is to produce a vaccine that can be given orally, and we therefore immunized mice by the oral route. For comparison, we also evaluated immunity in mice immunized by the intranasal route. Surprisingly, we found that maternal immunization enhanced the protective efficacy of the vaccine when it was administered by either route.
MATERIALS AND METHODS
Bacterial strains, media, growth conditions, and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. LB broth (4) and agar were used as complex rich media for the propagation of all bacterial strains. For animal experiments, χ9558(pYA4088) and χ9558(pYA3493) were grown and prepared as previously described (27). To determine the titer, serial dilutions of the RASV strains were plated onto MacConkey agar supplemented with 1% lactose, 0.05% arabinose, and 0.2% mannose. Some mice were inoculated with buffer only as controls. S. pneumoniae WU2 was cultured on brain heart infusion (BHI) agar containing 5% sheep blood or in Todd-Hewitt broth plus 0.5% yeast extract (8). WU2 is not adapted to enable colonization of the murine nasopharynx, so it is only used for intraperitoneal challenges.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli χ7213 | thi-1 thr-1 leuB6 glnV44 fhuA21 lacY1 recA1 ΔasdA4 Δ(zhf-2::Tn10) RP4-2-Tc::Mu[λ pir] | 39 |
| S. Typhimurium | ||
| χ3761 | Wild-type UK-1 | 14 |
| χ9558 | Δpmi-2426 Δ(gmd-fcl)-26 ΔPfur81::TT araC PBADfur ΔPcrp527::TT araC PBADcrp ΔasdA27::TT araC PBADc2 ΔaraE25 ΔaraBAD23 ΔrelA198::araC PBADlacI TT ΔsopB1925 ΔagfBAC811 | 6 |
| S. pneumoniae WU2 | Wild type, virulent, encapsulated, type 3 | 8 |
| Plasmids | ||
| pYA3493 | Asd+ pBRori β-lactamase signal sequence-based periplasmic secretion plasmid | 6 |
| pYA4088 | 852-bp DNA encoding the α-helical region of PspA from aa 3 to 285 in pYA3493 | 49 |
Mice.
BALB/c mice (8 weeks old) purchased from Charles River Laboratories (Wilmington, MA) were bred to produce pups. Some groups of female BALB/c mice (8 weeks old) were immunized orally with 20 μl containing 1 × 109 CFU of χ9558(pYA4088) suspended in buffered saline with gelatin (BSG) 2 weeks before breeding. All animal procedures were approved by the Arizona State University Animal Care and Use Committee. Mouse breeding cages were checked daily; new births were recorded, and the pups were kept with their mothers until weaning at the age of 3 weeks. Experimental groups contained two litters for safety and colonization experiments and three litters for immunogenicity experiments.
Safety of χ9558(pYA4088) in neonatal mice.
To assess safety in infant and neonatal mice, one litter per group (0-day-old [<24 h], 2-day-old, 4-day-old, and 7 day-old groups) from naïve mothers were orally inoculated with 5 μl containing approximately 5 × 108 CFU of χ9558(pYA4088). Health and the number of deaths were monitored and recorded every day for 6 weeks. This experiment was repeated three times.
Colonization of neonatal mice by χ9558(pYA4088).
For colonization studies, 0-, 2-, 4-, and 7-day-old pups (6/group) born to either naïve or immunized mothers were orally inoculated with 5 μl containing approximately 5 × 108 CFU of χ9558(pYA4088). Mice were euthanized on days 3 and 7 postinfection, and samples of the entire upper intestinal tract (ileum, jejunum, and duodenum), spleen, and liver were collected. Note that we sampled the entire small-intestinal tract because gut-associated lymphoid tissues (GALT), such a Peyer's patches, are not visually detectable in newborn mice. Tissues were weighed and homogenized in a total volume of 1 ml BSG. Serial dilutions were plated onto MacConkey agar plates containing 1% lactose, 0.05% arabinose, and 0.2% mannose to determine the number of viable bacteria. Plates were incubated at 37°C for at least 18 h. We also inoculated 900 μl of homogenized tissues into 5 ml selenite broth (Difco) for Salmonella enrichment. Samples that were negative by direct plating and positive by enrichment were recorded as 10 CFU/g. Samples that were negative both by direct plating and by enrichment were recorded as 0 CFU/g.
Immunogenicity studies.
Approximately 1 week before delivery, sera were collected from pregnant mice. All immunized mothers had reciprocal PspA and Salmonella lipopolysaccharide (LPS)-specific serum IgG titers ranging from 800 to 1,200 for individual mice. Male and female mice from each litter were placed in separate cages after weaning.
Neonatal (7-day-old) and infant (21-day-old) mice from either naïve or immunized mothers were immunized orally or i.n. with approximately 5 × 108 CFU of χ9558(pYA4088) administered in a 5.0-μl volume (2.5 μl/naris for the intranasal route) (36). Groups of mice from naïve mothers were also inoculated with the control strain χ9558(pYA3493), which does not express pspA. For intranasal immunizations, anesthesia was avoided in order to limit aspiration into the lungs (19). Mice were boosted with the same vaccine dose and by the same route at 3 and 6 weeks post-primary immunization for oral immunizations and at 2, 4, and 6 weeks post-primary immunization for i.n. groups. Age-matched mice inoculated with sterile BSG were used as controls for both groups. Preimmune sera were obtained by bleeding neonates and infants by mandibular vein puncture prior to vaccination. Further bleedings were performed at 3 and 6 weeks, and at biweekly intervals thereafter. Whole blood was incubated at 37°C for 60 min, and the clot was pelleted by centrifugation. The serum was removed from the whole blood and stored at −80°C. Vaginal wash samples were collected at 8 weeks after the first immunization and were stored at −80°C.
Antigen preparation.
S. Typhimurium LPS was purchased from Sigma. Outer membrane proteins (OMPs) and recombinant PspA (rPspA) protein were purified as described previously (23). The rPspA clone encoding the α-helical region of PspA (amino acids [aa] 1 to 302) in pET20b was a kind gift from Susan Hollingshead at the University of Alabama at Birmingham.
ELISA.
Sera from all mice in a group were pooled for analysis. An enzyme-linked immunosorbent assay (ELISA) was used to measure IgG antibodies against serovar Typhimurium LPS and rPspA in serum and IgG1 and IgG2a in serum and IgA in vaginal washes against rPspA as previously described (23). Absorbance was recorded at 405 nm using an automated ELISA plate reader (model EL311SX; Biotek, Winooski, VT). Absorbance readings that were 0.1 unit higher than phosphate-buffered saline (PBS) control values were considered positive. We did not measure maternal antibody titers in newborn mice or their rate of decline, since these would not likely contribute to the antibody titers in immunized infant mice, which were first measured just after weaning for mice immunized at the age of 7 days and 2 weeks or more after weaning for all other groups of mice.
IL-4 and IFN-γ ELISPOT assays.
At week 7, spleen cells were harvested from 6 mice per group. Cells from each spleen were assayed by an enzyme-linked immunospot (ELISPOT) assay in triplicate wells as previously described (41). Briefly, polyvinylidene difluoride (PVDF) membrane plates (Millipore) were first washed with sterile H2O and then coated with 100 μl of monoclonal antibodies (MAbs) against interleukin-4 (IL-4) or gamma interferon (IFN-γ) (BD PharMingen) at 5 μg/ml in PBS overnight at 4°C. The wells were washed with PBS and blocked with RPMI containing 10% fetal calf serum (FCS). Then 50 μl of cell medium (RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 IU/ml penicillin, 10 μg/ml streptomycin, and 1% HEPES) and 50 μl of spleen cells (100,000 per well) in cell medium with or without stimulation with rPspA at 5 μg/ml were added per well and incubated in the plates overnight under 5% CO2 at 37°C. The next day, the cell suspensions were discarded, and the plates were washed with PBS-0.05% Tween 20 (PBS-T). A biotinylated anti-IL-4 or anti-IFN-γ MAb (BD PharMingen) at 0.5 μg/ml in PBS-T with 1% FCS was added and incubated at room temperature for 2 h. After a wash with PBS-T, 100 μl/well of avidin peroxidase diluted 1:1,000 (vol/vol) in PBS-T containing 1% FCS was added, followed by incubation for 1 h at room temperature. After a wash with PBS-T, 100 μl of 3-amino-9-ethylcarbazole was added per well. Spots were developed for 15 min at room temperature. Plates were dried and analyzed by using an automated CTL ELISPOT reader system (Cellular Technology).
Pneumococcal challenge.
Four weeks after the final immunization, mice were challenged by intraperitoneal injection with 2 × 103 CFU (10 LD50s; for orally immunized mice) or 4 × 103 CFU (20 LD50s; for intranasally immunized mice) of S. pneumoniae WU2 in 100 μl of BSG (33). Challenged mice were monitored daily for 15 days.
Statistical analysis.
Data are presented as the geometric means and standard deviations in all assays. Mann-Whitney U test software (version 5.0; GraphPad Software, Inc.) was used for comparing the distributions of χ9558(pYA4088) in the tissues of neonatal mice. Analysis of variance (ANOVA) (SPSS Software), followed by Fisher's LSD (least significant difference) test, was used to evaluate differences in antibody titers and cytokine-secreting cell responses, which were determined with 95% confidence intervals. The Kaplan-Meier method (SPSS Software) was applied to obtain the survival fractions following i.p. challenge of orally immunized mice. A P value of <0.05 was considered statistically significant.
RESULTS
Safety of strain χ9558(pYA4088) in neonatal mice from naïve mothers.
To further assess the safety of χ9558(pYA4088) as reported elsewhere (16a), 155 neonatal mice ranging in age from 0, 2, 4 and 7 days were orally inoculated with 5 μl containing approximately 5 × 108 CFU of strain χ9558(pYA4088). Health and the number of deaths were monitored daily over a 6-week period. No disease symptoms, growth impairment, or death occurred at any time, confirming that χ9558(pYA4088) is completely safe in young mice.
Distribution of strain χ9558(pYA4088) in tissues of neonatal mice from either naïve or immunized mothers.
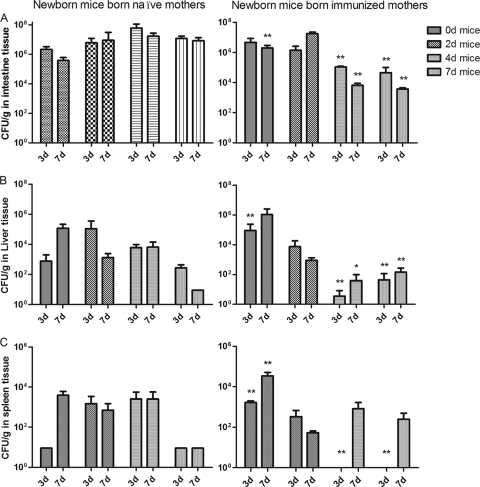
We examined the ability of χ9588(pYA4088) to colonize the intestine, liver, and spleen when administered to pups 0, 2, 4 or 7 days of age born to either naïve or immunized mothers. Tissue colonization was evaluated at 3 and 7 days after oral inoculation as described in Materials and Methods. Overall, immunization of the mother had the greatest effect on inhibiting colonization in pups inoculated at the age of 4 or 7 days but had no negative effect on pups inoculated at the age of 0 or 2 days (Fig. 1). Strain χ9558(pYA4088) colonized intestinal tissues to high levels in all groups (Fig. 1A). Despite the high level of intestinal colonization in the group of mice from naïve mothers inoculated at day 7, levels of colonization of the spleen and liver were somewhat lower than those in the other groups of mice from naïve mothers. Intestinal colonization was inhibited in pups from immune mothers that were immunized at the age of 4 or 7 days (P < 0.01) and was stimulated (on day 7) in pups immunized at day 0. Maternal immunization had a more profound effect on colonization of the liver and spleen (Fig. 1B and C). As with intestinal colonization, maternal immunity had no negative effect on pups inoculated at the age of 0 or 2 days, and for pups immunized on day 2, maternal immunity enhanced colonization at some time points. Among pups inoculated at the age of 4 days, liver colonization was inhibited in those from immunized mothers compared to that for pups from naïve mothers at both time points examined (Fig. 1B). For mice inoculated at day 0, maternal immunization resulted in higher numbers of χ9558(pYA4088) in the spleen on day 3 and day 7 (P < 0.01) (Fig. 1C). No vaccine was recovered from the spleens of pups from immune mothers 3 days after inoculation when they had been inoculated at the age of 4 or 7 days. However, by day 7 postinoculation, spleen colonization in these groups was similar to spleen colonization in mice from naïve mothers (Fig. 1C) (P < 0.05).
FIG. 1.
Distribution of S. Typhimurium strain χ9558(pYA4088) in tissues of newborn mice born to naïve or immunized mothers. Groups of pups were orally inoculated with χ9558(pYA4088) on the day (d) after birth indicated in the key. For mice born to naïve mothers, the doses were 1.4 × 108 CFU for 0-day-old mice, 1.6 × 108 CFU for 2-day-old mice, 3.0 × 108 CFU for 4-day-old mice, and 3.5 × 108 CFU for 7-day-old mice. For mice born to immunized mothers, the doses were 1.5 × 108 CFU for 0-day-old mice, 1.5 × 108 CFU for 2-day-old mice, 2.0 × 108 CFU for 4-day-old mice, and 1.0 × 108 CFU for 7-day-old mice. Significant differences between results obtained from mice born to naïve or immunized mothers are indicated (*, P < 0.01; **, P < 0.05). Tissue samples were taken from 3 mice/group on days 3 and 7 (3d and 7d) after inoculation. The results from three experiments are summarized.
Strain χ9558(pYA4088) is immunogenic in infant and neonatal mice from naïve or immunized mothers.
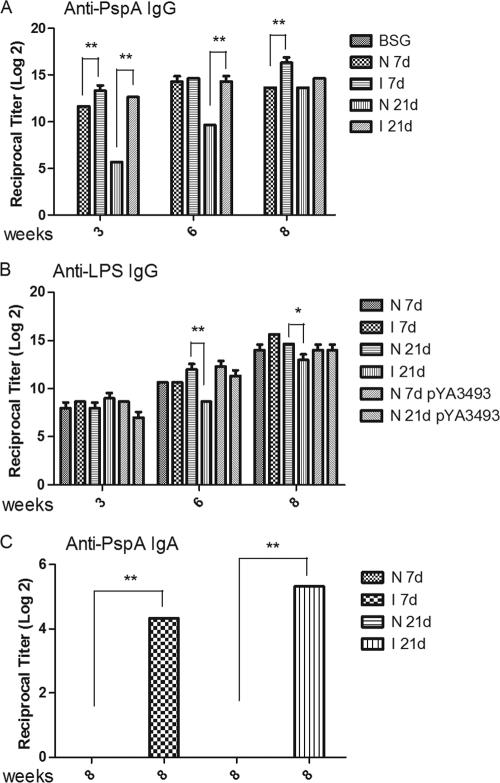
To assess the immune responses to rPspA after immunization in early life, 18 to 24 neonatal (7-day-old), and infant (21-day-old) mice per group from naïve or immunized mothers were orally immunized with approximately 5 × 108 CFU of χ9558(pYA4088) or the control strain χ9558(pYA3493). For convenience, we refer to these groups below as N 7d (naïve mother, pups immunized at day 7), I 7d (immunized mother, pups immunized at day 7), N 21d (naïve mother, pups immunized at day 21), and I 21d (immunized mother, pups immunized on day 21). Mice were immunized again 3 and 6 weeks following the first dose. Age-matched control mice were given sterile BSG and served as nonimmunized controls. Serum IgG antibody responses to rPspA and Salmonella LPS and mucosal IgA responses to PspA were measured. This experiment was performed twice with similar results, which have been pooled for analysis (Fig. 2).
FIG. 2.
ELISA measurements of serum IgG and mucosal IgA responses in immunized mice. (A and B) Serum IgG responses against rPspA (A) and S. Typhimunium LPS (B) were measured using pooled sera from neonates and infants born to either naïve (N) or immunized (I) mothers. (C) Mucosal IgA responses against rPspA were measured in pooled vaginal washes. Mice were immunized orally with either χ9558(pYA4088) (pspA) or χ9558(pYA3493) (control) or were mock immunized with BSG on either day 7 (7d) or day 21 (21d) after birth. Only mice from naïve mothers were inoculated with χ9558(pYA3493). Mice were boosted 3 and 6 weeks after the primary immunization. Error bars represent differences between triplicate wells. Significant differences between groups are indicated (*, P < 0.05; **, P < 0.01). No immune responses to PspA were detected in mice immunized with χ9558(pYA3493). No antibody to PspA or LPS was detected in mice inoculated with buffer only or in preimmune sera from vaccinated mice (reciprocal titer, <1:50).
The anti-PspA serum responses in mice from immunized mothers were higher at 3 weeks post-primary immunization than the responses in mice born to naïve mothers (Fig. 2A) (P < 0.01). The differences in IgG responses between pups from naïve and immunized mothers were greatest for the pups immunized at 21 days. The anti-PspA titers in pups from naïve mothers were slower to develop than titers in pups from immune mothers, although by week 8 there was no significant difference between the two groups. Among the N 7d and I 7d groups, pups from immunized mothers (I 7d) developed significantly higher titers than pups from naïve mothers (N 7d) by week 8. Overall, maternal immunity did not play a significant role in the development of serum anti-LPS IgG (Fig. 2B), except for the I 21d group, which had significantly lower titers than the other groups (P < 0.01 at 6 weeks; P < 0.05 at 8 weeks).
Mice have a common mucosal system (30), and vaginal responses are representative of responses in the nasopharyngeal tract (47). Therefore, we used vaginal washes to evaluate mucosal responses. This also allowed us to keep the mice alive for challenge studies. At week 8, vaginal washes were collected from the 12 to 17 female mice per group and were evaluated. No mucosal samples were taken from the remaining male mice. The development of mucosal IgA responses was dramatically and significantly enhanced by maternal immunity (Fig. 2C). There was no detectable anti-PspA IgA in either group of mice from naïve mothers, while mice from immune mothers developed a detectable IgA response (P < 0.01).
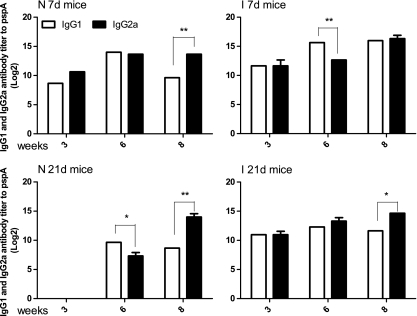
IgG isotype analyses of orally immunized mice.
The serum immune responses to rPspA were further examined by measuring the levels of IgG isotype subclasses IgG1 and IgG2a (Fig. 3). At the 3-week time point, the IgG1 and IgG2a levels were similar in all groups, indicating a balanced Th1-Th2 response, except for the N 21d group. For that group, the total IgG titers were too low to generate meaningful IgG1/IgG2a data. At 6 weeks, the I 7d and N 21d groups had developed a Th2 bias (IgG2a/IgG1 ratios of 0.125 and 0.25, respectively). By 8 weeks, after the second boost, the I 7d and I 21d groups had developed a balanced Th1-Th2 response trend, while the N 7d and N 21d groups had developed a Th1 bias (IgG2a/IgG1 ratios, 16 for the N 7d group and 32 for the N 21d group).
FIG. 3.
Serum IgG2a and IgG1 responses to rPspA measured by ELISA. The data represent IgG2a and IgG1 subclass antibody levels to rPspA in pooled sera from neonates and infants orally immunized with χ9558(pYA4088) at various times after immunization. Error bars represent differences between triplicate wells. Differences between IgG1 and IgG2a responses are indicated (*, P < 0.05; **, P < 0.01).
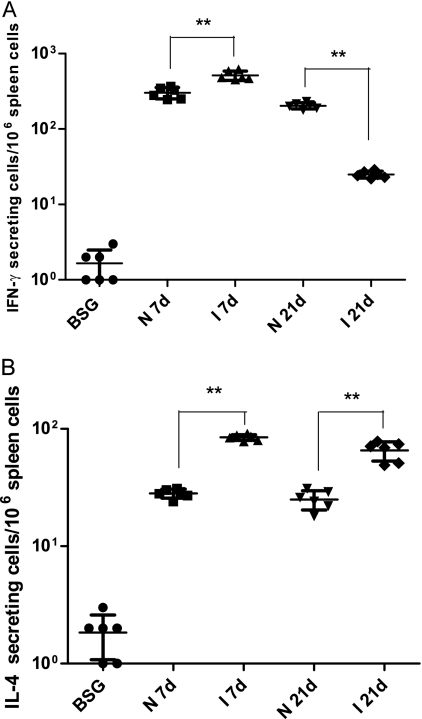
Antigen-specific stimulation of IL-4 or IFN-γ production.
To further evaluate the Th1/Th2 immune responses, we examined whether mice immunized as neonates and infants developed cell-mediated immunity to vaccine antigens. ELISPOT assays were used to compare PspA stimulation of IFN-γ (Th1 associated) and IL-4 (Th2 associated) production by spleen cells taken from immunized and control mice at week 7. The number of PspA-specific IFN-γ-secreting cells (Fig. 4A) in immunized neonates and infants born to either naïve or immunized mothers was significantly higher than that for the BSG control group (P < 0.01), except for the I 21d group, whose results were not significantly different from those of the BSG group (P > 0.05).
FIG. 4.
PspA-specific cytokine stimulation in mice immunized with χ9558(pYA4088) and in nonimmunized mice. Numbers of IFN-γ (A)- and IL-4 (B)-producing cells were determined by ELISPOT assays. Splenectomies were performed on euthanized mice 7 days after the final immunization. Mice mock immunized with BSG were included as controls. Splenocytes were harvested from 6 mice per group, and cells from each spleen were assayed in triplicate. Each symbol represents the results from a single well. The results from each well are expressed as ELISPOTs per million splenocytes minus any background ELISPOTs from unpulsed cells from mock-immunized controls. Significant differences between groups are indicated (**, P < 0.01).
The number of PspA-specific IFN-γ-secreting cells in mice born to immunized mothers was significantly greater than that in mice born to naïve mothers (P < 0.05) except for mice immunized at the age of 21 days, for whom the number of IFN-γ-secreting cells in mice from immunized mothers was lower than the number in mice from naïve mothers (P < 0.01).
For both the day-7 and day-21 groups, the numbers of PspA-specific IL-4-secreting cells were higher in mice born to immunized mothers than in mice born to naïve mothers (P < 0.01). The numbers of IL-4-secreting cells in mice from naïve mothers and from immunized mothers were significantly different from those in BSG controls (P < 0.05).
Evaluation of protective immunity.
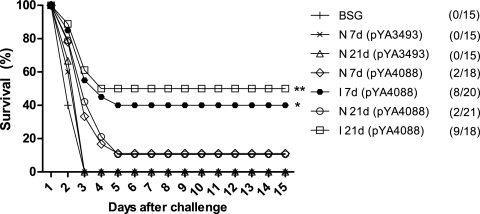
To evaluate the capacity of χ9558(pYA4088) to protect mice immunized as neonates or infants, immunized mice (18 to 24 mice per group) were challenged intraperitoneally with 2 × 103 CFU (10 LD50s) of S. pneumoniae WU2 4 weeks after the final boost (≥11 weeks of age) (Fig. 5). All mice inoculated with χ9558(pYA3493), a Salmonella strain that does not express pspA, or with BSG succumbed to the infection within 3 days. All groups of mice immunized with χ9558(pYA4088) were significantly protected from challenge compared to controls (P < 0.05). Protection was significantly greater in the I 21d group than in the N 21d group (P < 0.01), and protection in the I 7d group was significantly greater than that in the N 7d group (P < 0.05), indicating that maternal immunization enhances the protective efficacy of χ9558(pYA4088).
FIG. 5.
Oral immunization with χ9558(pYA4088) protects BALB/c mice against i.p. challenge with S. pneumoniae WU2. The survival of orally immunized or nonimmunized mice after intraperitoneal challenge with 2 × 103 CFU of S. pneumoniae WU2 4 weeks after the final immunization is shown. Each experiment was performed twice with similar results, and the data have been pooled for presentation and analysis. All vaccine groups were significantly different from the vector control [χ9558(pYA3493)] and the PBS control (P < 0.01). **, P < 0.01 for survival of infants born to naïve mothers compared to that of infants born to immunized mothers; *, P < 0.05 for survival of neonates born to naïve mothers compared to that of neonates born to immunized mothers.
Immunogenicity and protective efficacy of χ9558(pYA4088) when administered intranasally.
To evaluate the vaccine when administered intranasally, we performed intranasal immunizations of 7- and 21-day-old mice, with 3 additional immunizations performed at weekly intervals. Even though the data were as good or better than those for oral immunization, we have not presented the actual data here, since intranasal immunization with live Salmonella vaccines is highly unlikely, in view of our data demonstrating the access of these vaccines to the brain when intranasally administered (6). The serum IgG titers against LPS and PspA were about 2- to 6-fold higher in mice immunized intranasally (data not shown) than in mice immunized orally, but the trends were essentially the same. The anti-PspA serum IgG response developed more quickly in mice from immunized mothers, but titers were similar by 8 weeks after the primary immunization. Vaginal mucosal IgA responses were significantly higher in mice from immunized mothers, and were detectable earlier after primary immunization, than in mice from naïve mothers. Also, the mucosal IgA titers were 2 to 4 times higher after intranasal immunization than after oral immunization. The IgG1 and IgG2a analysis indicated that all mice developed a balanced Th1-Th2 response, regardless of maternal immune status or the age of primary immunization, although by 8 weeks, there was a Th1 bias in mice immunized at the age of 7 days from both naïve (IgG2a/IgG1 ratio, 4; P < 0.01) and immunized (IgG2a/IgG1 ratio, 2; P < 0.05) mothers (data not shown). Finally, protection against S. pneumoniae challenge also showed a similar trend. Pups from immunized mothers were significantly better protected than pups from naïve mothers (see Fig. S1 in the supplemental material). The overall level of protection was greater in the intranasally immunized group than in the orally immunized group.
DISCUSSION
One of the primary goals of our research is to develop an oral Salmonella enterica serovar Typhi-vectored vaccine to protect infants from pneumonia and other diseases caused by S. pneumoniae. A critical first step in attaining this goal is to develop a Salmonella vector that is safe enough to be administered to infants and young children while retaining the ability to elicit a strong immune response against the vectored antigen(s). S. Typhi causes typhoid fever in humans, and S. Typhimurium causes a similar disease in mice (35). Since S. Typhi is a host-restricted species, we first set about developing an attenuation strategy for S. Typhimurium. While the two Salmonella serovars do not interact with their hosts in precisely the same way, the interactions are similar enough to allow us to test various iterations of attenuation and immunity-enhancing strategies in mice using S. Typhimurium. The culmination of our previous work was the construction of strain χ9558 (16a), which carries 10 mutations rationally designed to enhance immunogenicity (27, 28) while providing a high level of safety to the immunized host (6, 16a).
Previous work showed that χ9558 (carrying one of several different pspA-encoding plasmids) is safe (6) and immunogenic (27) in adult mice and that χ9558(pYA4088) is safe in young mice ranging in age from the day of birth to 7 days (16a). In this work, we confirmed the safety of χ9558(pYA4088) with an additional 155 neonatal mice, ranging in age from the day of birth to 7 days. This strain, while safe, was able to colonize the intestinal tracts, livers, and spleens of young mice (Fig. 1). However, we should point out that since the entire small intestine was sampled, we cannot be certain that GALT was actually colonized. Lack of efficient GALT colonization could explain the colonization results for mice from naïve mothers inoculated at the age of 7 days. For these mice, the numbers of χ9558(pYA4088) organisms observed in the intestinal tracts at days 3 and 7 postimmunization were similar to the numbers of χ9558(pYA4088) organisms observed at days 3 and 7 postimmunization in the intestinal tracts of mice from naïve mothers that were inoculated at the age of 0, 2, or 4 days. However, the numbers of χ9558(pYA4088) organisms that reached the spleens and livers of mice from naïve mothers inoculated at the age of 7 days were 1 to 2 log units lower than the numbers of χ9558(pYA4088) organisms that reached the spleens and livers of mice in the other three groups at both sampling times. Since the GALT is the portal to these deeper tissues, it is likely that efficient GALT colonization was not achieved in these mice. We thus could be detecting bacteria that have attached to, but failed to invade, the GALT.
Immunization of infants can be compromised by maternal antibodies (11, 33, 35), a matter of concern when one is designing a vaccine for infants. Among pups from immunized mothers, colonization by the vaccine strain was delayed in mice immunized on day 4 or 7 after birth but, surprisingly, not in mice immunized on day 0 or 2 after birth. One explanation for these results is that the observed delay in colonization may have been due to anti-Salmonella antibodies in the milk from the immunized mothers (17, 46). This interpretation is based on the assumption that the day-0 and day-2 mice did not ingest an adequate amount of anti-Salmonella secretory IgA (sIgA) to inhibit colonization. Alternatively, these results could be due to the lack of development of the immune system in 0- and 2-day-old mice. However, in spite of the delay in colonization, by day 7 after immunization, χ9558(pYA4088) had colonized all tissues examined in mice from both immunized and naïve mothers.
Strain χ9558(pYA4088) induced a strong immune response to PspA in neonates and infants from either naïve or immunized mothers (Fig. 2). Interestingly, despite the initial delay in colonization, serum anti-PspA antibody responses were significantly higher in neonates and infants from immunized mothers than in neonates and infants from naïve mothers at weeks 2 and 4 postimmunization, indicating that maternal antibodies either enhanced the development of or contributed to the serum immune response. This difference was abrogated after the second boost at week 6, leading to similar titers in all groups. However, the mucosal IgA titers were significantly higher in mice from immunized mothers than in those from naïve mothers at week 8. These collective results were the opposite of what we expected, and we have no definitive explanation for these results, which are, however, an added beneficial feature of our vaccine development strategy.
The innate immune system modulates the quantity and quality of long-term T- and B-cell memory and the protective immune response to pathogens (37). Neonatal immature CD4+ T cells often polarize toward a Th2 rather than a Th1 pattern upon immunization (15, 38), and a weak Th2-biased response is observed after neonatal immunization with conjugate vaccines (20). Recently, Capozzo et al. demonstrated that immunization of neonatal mice with a Salmonella vaccine synthesizing the C fragment of tetanus toxin resulted in a mixed Th1-Th2 response to the antigen before boosting at the age of 22 days and a strong Th2 response after the boost, by the age of ca. 9 weeks (11). In our study, we observed a Th1 bias in neonates born to naïve mothers and a balanced Th1-Th2 response in mice from immunized mothers before boosting (at the 2-week time point) (Fig. 3). After the second immunization (3 weeks), the N 7d group developed a balanced Th1-Th2 response while the I 7d group developed a Th2 bias. After the third immunization (6 weeks), the N 7d group developed a Th1 bias while the I 7d group developed a balanced response. The Th1 bias predicted by the IgG2a/IgG1 ratios for the N 7d group (Fig. 3) is consistent with the relative numbers of IFN-γ- and IL-4-secreting cells (Fig. 4), while the data in Fig. 4 would indicate a Th1 bias for the I 7d group despite the apparently balanced Th1-Th2 response predicted from the data in Fig. 3.
Strong PspA-specific mucosal IgA responses were detected in vaginal washes in neonates and infants born to immunized mothers after the final boost. In contrast, no IgA antibody was detected in immunized neonates or infants born to naïve mothers (Fig. 2C). In mice, the generation of IgA-specific B cells depends on Th2 cell-directed synthesis of IL-4 and IL-5 (2, 3, 26, 32). Therefore, the reduced mucosal IgA response in neonates born to naïve mothers could be due to poor stimulation of Th2 cells. This argument is consistent with the low levels of IL-4-secreting cells in the spleens of these mice (Fig. 4B). In other studies, we have demonstrated that for older mice, oral immunization can prevent colonization of the nasopharynx by selected pneumococcal strains and thus confer complete protection against septicemic disease (48).
These data demonstrate that immunization with χ9558(pYA4088) can generate strong B- and T-cell responses in very early life and elicit a stronger cell-mediated immune response against PspA when the mother has been immunized. Our results showing that χ9558(pYA4088) can elicit mucosal and systemic responses in neonates and young infants are highly encouraging. However, the level of protection observed in our experiments is lower than what we have seen in older mice. In a previous study, adult mice orally immunized and boosted with χ9558 expressing pspA were protected from a 200-LD50 challenge dose of S. pneumoniae WU2 (27). Therefore, improvements still need to be made in order to achieve our goal of providing an oral RASV for infants. We are currently evaluating a number of additional antigens for inclusion in our vaccine, including PspC (49). The construction of a vaccine strain expressing multiple antigens (e.g., PspA and PspC), coupled with additional improvements to the Salmonella carrier, should enhance the protective efficacy of this vaccine in neonates and infants.
Here we demonstrate for the first time, with newborn and infant mice, that a RASV expressing a pneumococcal gene can stimulate the neonatal and infant immune systems. Despite the presence of maternal antibodies, χ9558(pYA4088) induced protective anti-PspA immune responses, resulting in higher protective efficacy against pneumococcal challenge. Thus, this strategy may be useful for establishing early-life protection against pneumococcal disease.
Supplementary Material
Acknowledgments
This research was supported by grant 37863 from the Bill & Melinda Gates Foundation.
We thank Susan K. Hollingshead and David Briles of the University of Alabama at Birmingham for providing pneumococcal strains, Yuhua Li and Wei Xin for suggestions on some experiments, Vidya Ananthnarayan and Qing Liu for protein purification, and Jacquelyn Kilbourne for assistance with animal experiments.
Footnotes
Published ahead of print on 6 January 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Beagley, K. W., J. H. Eldridge, H. Kiyono, M. P. Everson, W. J. Koopman, T. Honjo, and J. R. McGhee. 1988. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J. Immunol. 141:2035-2042. [PubMed] [Google Scholar]
- 3.Beagley, K. W., J. H. Eldridge, F. Lee, H. Kiyono, M. P. Everson, W. J. Koopman, T. Hirano, T. Kishimoto, and J. R. McGhee. 1989. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J. Exp. Med. 169:2133-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, S., H. Shinefield, R. Cohen, D. Floret, J. Gaudelus, C. Olivier, and P. Reinert. 2004. Clinical effectiveness of seven-valent pneumococcal conjugate vaccine (Prevenar) against invasive pneumococcal diseases: prospects for children in France. Arch. Pediatr. 11:843-853. [DOI] [PubMed] [Google Scholar]
- 6.Bollen, W. S., B. M. Gunn, H. Mo, M. K. Lay, and R. Curtiss III. 2008. Presence of wild-type and attenuated Salmonella enterica strains in brain tissues following inoculation of mice by different routes. Infect. Immun. 76:3268-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles, D. E., S. Hollingshead, A. Brooks-Walter, G. S. Nabors, L. Ferguson, M. Schilling, S. Gravenstein, P. Braun, J. King, and A. Swift. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707-1711. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858-867. [DOI] [PubMed] [Google Scholar]
- 9.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell, G. D., Jr., and R. Silberman. 1998. Drug-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 26:1188-1195. [DOI] [PubMed] [Google Scholar]
- 11.Capozzo, A. V. E., L. Cuberos, M. M. Levine, and M. F. Pasetti. 2004. Mucosally delivered Salmonella live vector vaccines elicit potent immune responses against a foreign antigen in neonatal mice born to naïve and immune mothers. Infect. Immun. 72:4637-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtiss, R., III, S. Y. Wanda, B. M. Gunn, X. Zhang, S. A. Tinge, V. Ananthnarayan, H. Mo, S. Wang, and W. Kong. 2009. Salmonella enterica serovar Typhimurium strains with regulated delayed attenuation in vivo. Infect. Immun. 77:1071-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtiss, R., III, X. Zhang, S. Y. Wanda, H. Y. Kang, V. Konjufca, Y. Li, B. Gunn, S. Wang, G. Scarpellini, and I. S. Lee. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p. 297-313. In K. A. Brogden, N. Cornick, T. B. Stanton, Q. Zhang, L. K. Nolan, and M. J. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC.
- 14.Curtiss, R., III, S. B. Porter, M. Munson, S. A. Tinge, J. O. Hassan, C. Gentry-Weeks, and S. M. Kelly. 1991. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry, p. 169-198. In L. C. Blankenship, J. S. Bailey, N. A. Cox, S. E. Craven, R. J. Meinersmann, and N. S. Stern (ed.), Colonization control of human bacterial enteropathogens in poultry. Academic Press, Inc., San Diego, CA.
- 15.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of TH1 and TH2 immunity in neonatal mice. Science 271:1728-1730. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood, B. 1999. The epidemiology of pneumococcal infection in children in the developing world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Gunn, B. M., S.-Y. Wanda, D. Burshell, C. Wang, and R. Curtiss III. Construction of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17:354-362. [DOI] [PMC free article] [PubMed]
- 17.Hanson, L. A. 1998. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 81:523-533. [DOI] [PubMed] [Google Scholar]
- 18.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346-1354. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen, H., S. Bjarnarson, G. Del Giudice, M. Moreau, C.-A. Siegrist, and I. Jonsdottir. 2002. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 70:1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen, H., S. Hannesdottir, S. P. Bjarnarson, D. Schulz, E. Trannoy, C. A. Siegrist, and I. Jonsdottir. 2006. Early life T cell responses to pneumococcal conjugates increase with age and determine the polysaccharide-specific antibody response and protective efficacy. Eur. J. Immunol. 36:287-295. [DOI] [PubMed] [Google Scholar]
- 21.Kang, H. Y., and R. Curtiss III. 2003. Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol. Med. Microbiol. 37:99-104. [DOI] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Kang, H. Y., J. Srinivasan, and R. Curtiss III. 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein, J. O. 1981. The epidemiology of pneumococcal disease in infants and children. Rev. Infect. Dis. 3:246-253. [DOI] [PubMed] [Google Scholar]
- 25.Kong, W., S. Y. Wanda, X. Zhang, W. Bollen, S. A. Tinge, K. L. Roland, and R. Curtiss III. 2008. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc. Natl. Acad. Sci. U. S. A. 105:9361-9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebman, D. A., and R. L. Coffman. 1988. The effects of IL-4 and IL-5 on the IgA response by murine Peyer's patch B cell subpopulations. J. Immunol. 141:2050-2056. [PubMed] [Google Scholar]
- 27.Li, Y., S. Wang, G. Scarpellini, B. Gunn, W. Xin, S. Y. Wanda, K. L. Roland, and R. Curtiss III. 2009. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. U. S. A. 106:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y., S. Wang, W. Xin, G. Scarpellini, Z. Shi, B. Gunn, K. L. Roland, and R. Curtiss III. 2008. A sopB deletion mutation enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Infect. Immun. 76:5238-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link, C., T. Ebensen, L. Ständner, M. Déjosez, E. Reinhard, F. Rharbaoui, and C. A. Guzmán. 2006. An SopB-mediated immune escape mechanism of Salmonella enterica can be subverted to optimize the performance of live attenuated vaccine carrier strains. Microbes Infect. 8:2262-2269. [DOI] [PubMed] [Google Scholar]
- 30.McDermott, M. R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892-1898. [PubMed] [Google Scholar]
- 31.McGee, L., H. Wang, A. Wasas, R. Huebner, M. Chen, and K. P. Klugman. 2001. Prevalence of serotypes and molecular epidemiology of Streptococcus pneumoniae strains isolated from children in Beijing, China: identification of two novel multiply-resistant clones. Microb. Drug Resist. 7:55-63. [DOI] [PubMed] [Google Scholar]
- 32.Murray, P. D., D. T. McKenzie, S. L. Swain, and M. F. Kagnoff. 1987. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J. Immunol. 139:2669-2674. [PubMed] [Google Scholar]
- 33.Nayak, A. R., S. A. Tinge, R. C. Tart, L. S. McDaniel, D. E. Briles, and R. Curtiss III. 1998. A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect. Immun. 66:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paradiso, P. 2009. Essential criteria for evaluation of pneumococcal conjugate vaccine candidates. Vaccine 27(Suppl. 3):C15-C18. [DOI] [PubMed] [Google Scholar]
- 35.Pasetti, M. F., M. M. Levine, and M. B. Sztein. 2003. Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors. Vaccine 21:401-418. [DOI] [PubMed] [Google Scholar]
- 36.Pickett, T. E., M. F. Pasetti, J. E. Galen, M. B. Sztein, and M. M. Levine. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect. Immun. 68:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 38.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 39.Roland, K., R. Curtiss III, and D. Sizemore. 1999. Construction and evaluation of a Δcya Δcrp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 43:429-441. [PubMed] [Google Scholar]
- 40.Rudan, I., S. El Arifeen, R. E. Black, and H. Campbell. 2007. Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect. Dis. 7:56-61. [DOI] [PubMed] [Google Scholar]
- 41.Sedgwick, J. D., and P. G. Holt. 1983. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods 57:301-309. [DOI] [PubMed] [Google Scholar]
- 42.Siegrist, C.-A. 2007. The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 137:S4-S9. [DOI] [PubMed] [Google Scholar]
- 43.Siegrist, C.-A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 44.Singleton, R. J., J. C. Butler, L. R. Bulkow, D. Hurlburt, K. L. O'Brien, W. Doan, A. J. Parkinson, and T. W. Hennessy. 2007. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine 25:2288-2295. [DOI] [PubMed] [Google Scholar]
- 45.Tumpey, T. M., M. Renshaw, J. D. Clements, and J. M. Katz. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van de Perre, P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374-3376. [DOI] [PubMed] [Google Scholar]
- 47.Wu, H.-Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16:286-292. [DOI] [PubMed] [Google Scholar]
- 48.Xin, W., Y. Li, H. Mo, K. L. Roland, and R. Curtiss III. 2009. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 77:4518-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin, W., S. Y. Wanda, Y. Li, S. Wang, H. Mo, and R. Curtiss III. 2008. Analysis of type II secretion of recombinant pneumococcal PspA and PspC in a Salmonella enterica serovar Typhimurium vaccine with regulated delayed antigen synthesis. Infect. Immun. 76:3241-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Baumler, and L. G. Adams. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.