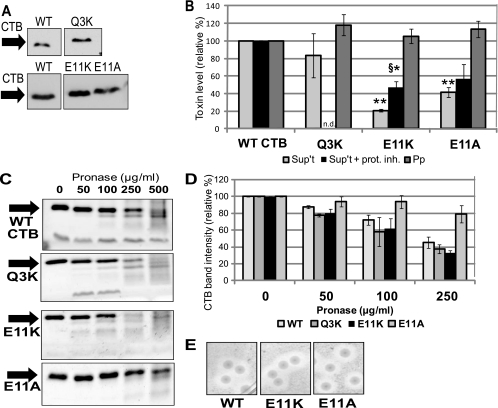
FIG. 3.
Mutation of Glu-11 impairs CTB secretion from V. cholerae. (A) Representative immunoblot of TCA-precipitated total culture samples, adjusted for CFU, showing induced expression of WT CTB, CTB[Q3K], CTB[E11K], and CTB[E11A] in strain P4. Blots were probed with anti-CT antibody. (B) Strains expressing WT CTB and the indicated CTB mutants were fractionated to isolate cell-free supernatant (Sup't) and periplasm (Pp). Each fraction was tested for pentamer levels by GM1 ELISA, with WT levels set to 100%. Supernatant levels were normalized to CFU, and periplasm levels were normalized to alkaline phosphatase activity. *, P < 0.05; **, P < 0.0005 compared to wild-type (n ≥ 3). For some experiments, cultures were grown in the presence of a protease inhibitor cocktail (+ prot. inh.). §, P < 0.05 compared to E11K supernatant levels without protease inhibitor (n ≥ 2). (C) Representative protein gels showing degradation of WT CTB or the indicated mutant pentamer. Purified pentamer (500 ng) was incubated with pronase at the indicated final concentration for 1 h at 37°C. Following the incubation, samples were boiled and separated by 15% SDS-PAGE, and the gel was stained with Ruby Red. (D) Densitometric measurements of the intensities of the CTB bands on the protein gels described in the legend for panel C. The intensity of the band corresponding to untreated WT or the indicated mutant CTB was set to 100% (n ≥ 2). (E) Zones of clearance formed by V. cholerae on skim milk agar. Strains expressing WT CTB and the indicated mutant pentamers were plated on skim milk agar and incubated for 36 h at 37°C.