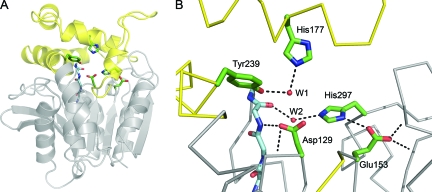
FIG. 6.
Cif active site. (A) Ribbon diagram of Cif-WT. The cap domain, consisting of residues 155 to 242, is colored yellow, and the core is in gray. The side chains of active-site residues are modeled as sticks, and the main chain of the HGFG motif is shown with the carbons colored light blue. (B) A detailed view of the active site. Hydrogen bonds are shown as dotted lines and the main chain as a Cα trace. The carboxylate of Glu153 participates in three hydrogen bonds. One is accepted from His297 and two from the protein backbone via the amide nitrogens of Gly266 and Met272. Asp129 is positioned by hydrogen bonds donated from the amide nitrogens of neighboring residue Ile130 and of Phe63 of the HGFG motif. W1 is coordinated by hydrogen bonds to Tyr239 and His177. W2 donates hydrogen bonds to the carbonyl oxygen of Phe63 and the imidazole of His297.