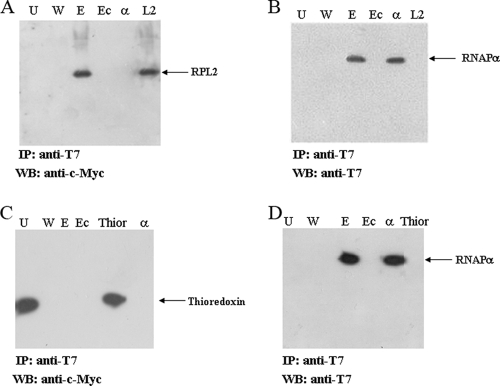
FIG. 3.
Coimmunoprecipitation experiments. Total protein extracts of E. coli strain C41 cotransformed with pET28a-αpol and pET22b-c-Myc-L2 and with pET28a-αpol and pET22b-c-Myc-Thioredoxin were subjected to immunoprecipitation with agarose-linked T7 antibody and with agarose beads alone as a control, followed by immunoblotting with anti-c-Myc antibody (A and C) and with anti-T7 antibody (B and D). In this experiment the recombinant RNAPα contained a T7 tag. In all immunoblots, lanes U, W, and E contained the unbound, wash, and elution fractions when anti-T7 antibody beads were used, respectively, while lane Ec contained the fraction eluted from agarose beads alone. (A and B) T7-RNAPα-c-Myc-L2 coimmunoprecipitation. Lane α contained the purified T7-tagged form of RNAPα, and lane L2 contained the purified c-Myc-tagged form of RPL2. (C and D) No T7-RNAPα-c-Myc-thioredoxin coimmunoprecipitation. Lane Thior contained the purified c-Myc-tagged form of thioredoxin, and lane α contained the purified T7-tagged form of RNAPα. IP, immunoprecipitation; WB, Western blotting.