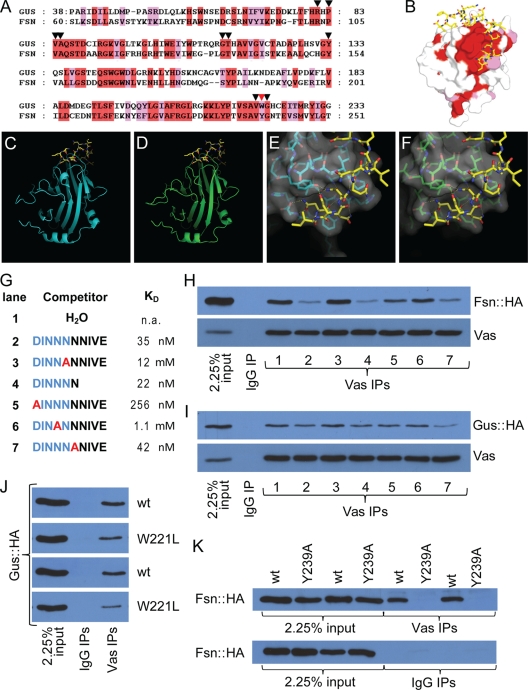
FIG. 1.
Gus and Fsn are Vas-interacting proteins. (A) Sequence alignment of the SPRY domains of Gus and Fsn. Identical and similar residues are highlighted in red and pink, respectively. Inverted triangles highlight the residues that form the Vas binding pocket. The red inverted triangle marks the residue W221 of Gus and Y239 of Fsn. (B) Surface view of a Gus crystal structure (PDB code 2IHS) (44). Surface residues conserved between Gus and Fsn are colored as described for panel A. (C to F) The B30.2/SPRY domains of Gus and Fsn are likely to form highly similar 3D structures. Panels C and E show crystal structures obtained from Gus (cyan) in complex with a Vas fragment (yellow) (PDB code 2IHS) (45). Panels D and F are structural predictions of the B30.2/SPRY domain of Fsn onto the crystals shown in panels C and E, respectively. The surface topology of the predicted Vas-binding site on Fsn (F) is almost identical to that of Gus (E). (G) Peptides used for the competition experiments shown in panels H and I. Kd values (apparent dissociation constants) for the interactions between Gus and peptides (45) are shown. (H) Fsn::HA and Vas coimmunoprecipitate. The interaction is sensitive to competition with peptides containing an intact DINNN motif. (I) GusL::HA and Vas coimmunoprecipitate. While the interaction is sensitive to peptide competition, the effects are much less pronounced than is the case for Fsn. (J) Both the wild type and W221L mutant Gus::HA associate with Vas in coimmunoprecipitation experiments; however, the amino acid substitution reduces the affinity of the interaction. All transgenic lines expressing GusL::HAW221L produced low levels of protein, possibly indicating a destabilizing effect of the substitution. Two transgenic lines are shown for Gus::HAW221L (second and fourth panels from the top), both compared to the same Gus::HA strain that expressed at a similar level to the mutant protein (first and third panels). (K) Unlike wild-type Fsn::HA, transgenic Fsn containing the Y239A mutation does not detectably coimmunoprecipitate with Vas. Two independent transgenic lines for each protein are shown.