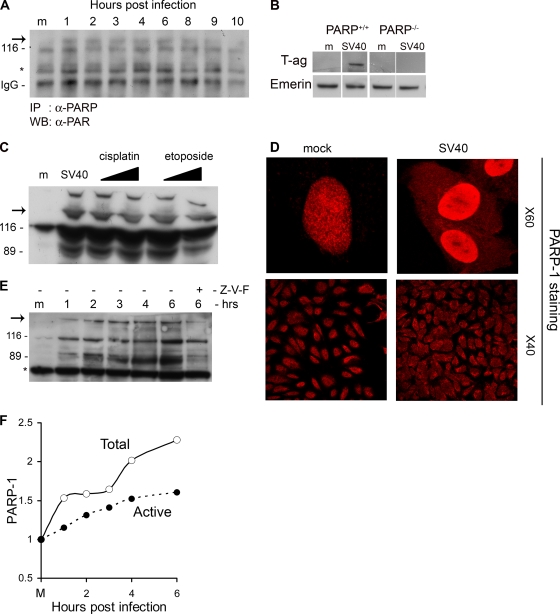
FIG. 1.
Activation of PARP-1 and Caspases. (A) PARP-1 was immunoprecipitated from total cell extracts at the designated time points with anti-PARP-1 antibodies. Western detection was performed with anti-PAR antibody. PARP-1 is 116 kDa. The arrow points to active, poly(ADP-ribosylated) PARP-1. IgG served as a loading control. (B) PARP-1 is required for the infection to proceed. PARP+/+ and PARP−/− cell lines were assayed for T-antigen expression 48 h after infection. Nuclear extracts were prepared, and T antigen was analyzed by Western blotting. Emerin detection served as a loading control. In this experiment we used an exceptionally high MOI of 500 because mouse cells are nonpermissive for productive SV40 infection as they do not support T-antigen-dependent viral DNA replication (9). (C) Cleavage by caspases. The Western blot shows the uncleaved 116-kDa protein and the 89-kDa caspase cleavage product. Etoposide and cisplatin were added at 15 and 30 μM. Western blotting was performed 48 h after the addition of etoposide and cisplatin and 6 h after infection by SV40. The arrow indicates active, poly(ADP-ribosylated) PARP-1. (D) Cellular distribution of PARP-1. Cells were fixed with 4% formaldehyde at 6 h postinfection and stained with polyclonal anti-PARP-1 and Cy3. Images in the top panels were taken at magnification of ×60 with zoom 3; lower images are at a magnification of ×40. Control and infected cells were at the same confluence. (E) Detection of active PARP-1 in total cell lysates. Western blotting was performed following loading of 20 μg of total protein per lane. The arrow points to higher-molecular-weight species of PARP-1 with extensive poly(ADP-ribosylation). The 89-kDa caspase cleavage product is significantly reduced in the presence of a 70 μM concentration of the pan-caspase inhibitor Z-VAD-FMK (Z-V-F). This experiment was reproduced five times. (F) Quantification of PARP-1 and active poly(ADP-ribosylated) PARP-1 following SV40 infection.