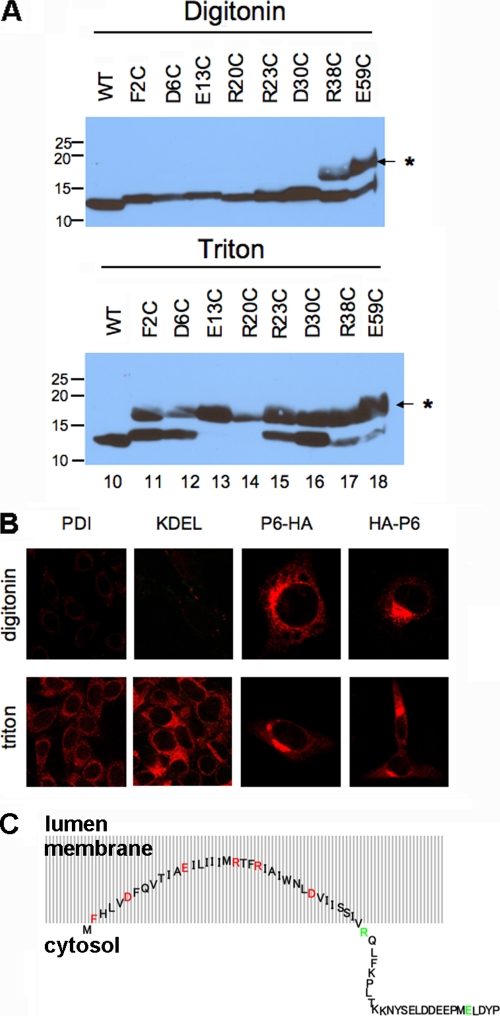
FIG. 2.
Residues 2 to 37 of p6 are membrane embedded with both termini in the cytoplasm. (A) Each charged residue within the N-terminal hydrophobic domain of p6-HA, as well as the Phe at position 2, was replaced singly with a cysteine and expressed in 293T cells. The cells were permeabilized with digitonin (top) or Triton X-100 (bottom) and then treated with maleimide PEG (MP) (5 kDa). SDS-PAGE was performed, followed by immunoblotting with anti-HA antibody. The positions of molecular mass standards (in kilodaltons) are indicated to the left of the gels. The position of the MP-modified band is indicated by the asterisk and small arrow to the right of the gels. WT, wild type. (B) 17Cl-1 cells were transfected with p6-HA or HA-p6 for 18 h and then fixed with 4% paraformaldehyde. The cells were permeabilized with either digitonin or Triton X-100 as indicated and then stained with anti-HA antibody (red). For both constructs, the HA tag could be detected with either method of permeabilization. Control experiments were performed by staining cells for two proteins in the lumen, PDI and KDEL. (C) Schematic diagram showing the putative conformation of p6 relative to intracellular membranes. Residues 2 to 37 are buried in the membrane, although the N-terminal amino acids are likely to be near the membrane/cytosol interface. Although drawn as an extended structure, the precise structure is not known, and p6 may actually form a hairpin. Mutated residues were located in the membrane (inaccessible to maleimide PEG [red]) or in the cytosol (accessible [green]).