Abstract
Viruses have evolved an assortment of mechanisms for regulating the Akt signaling pathway to establish a cellular environment more favorable for viral replication. Myxoma virus (MYXV) is a rabbit-specific poxvirus that encodes many immunomodulatory factors, including an ankyrin repeat-containing host range protein termed M-T5 that functions to regulate tropism of MYXV for rabbit lymphocytes and certain human cancer cells. MYXV permissiveness in these human cancer cells is dependent upon the direct interaction between M-T5 and Akt, which has been shown to induce the kinase activity of Akt. In this study, an array of compounds that selectively manipulate Akt signaling was screened and we show that only a subset of Akt inhibitors significantly decreased the ability of MYXV to replicate in previously permissive human cancer cells. Furthermore, reduced viral replication efficiency was correlated with lower levels of phosphorylated Akt. In contrast, the PP2A-specific phosphatase inhibitor okadaic acid promoted increased Akt kinase activation and rescued MYXV replication in human cancer cells that did not previously support viral replication. Finally, phosphorylation of Akt at residue Thr308 was shown to dictate the physical interaction between Akt and M-T5, which then leads to phosphorylation of Ser473 and permits productive MYXV replication in these human cancer cells. The results of this study further characterize the mechanism by which M-T5 exploits the Akt signaling cascade and affirms this interaction as a major tropism determinant that regulates the replication efficiency of MYXV in human cancer cells.
Following viral infection, substantial alterations in cellular physiology often lead to modification of various cellular pathways critical to the success of viral replication. The demands for energy, nutrients, and macromolecular synthesis that accompany viral replication can be substantial; thus, many viruses have evolved elaborate strategies for hijacking key cellular signaling networks necessary to support their demands (9). By the same token, antiviral pathways activated by the virus infection may also need to be blocked or subverted to ensure successful virus replication. Poxviruses possess large double-stranded DNA (dsDNA) genomes that encode multiple gene products that specifically modify or debilitate the various host signaling responses of the infected cell (28). Many of the immunoregulatory factors expressed by poxviruses have been well characterized, and these factors include virokines, viroreceptors, signaling modulators, and inhibitors of various antiviral responses, such as initiation of apoptosis pathways and signaling by protective cytokines, like interferon and tumor necrosis factor (TNF) (42).
Myxoma virus (MYXV) is a member of the Leporipoxvirus genus and exhibits a restricted pathogenesis that is limited to rabbits, primarily due to its specific immunomodulation of the immune system of leporids (48). In rabbits (Sylvilagus spp.) of the Americas, MYXV infection results in a benign infection, characterized by a cutaneous fibroma restricted to the site of inoculation (14); however, the same virus causes a rapid systemic and highly lethal infection called myxomatosis in European rabbits (Oryctolagus cuniculus) (15). Although MYXV has a narrow host range in nature and is pathogenic only to European rabbits, the tropism of MYXV has recently been extended to include human tumor cells in vitro (6, 47, 54, 57, 60) and in xenografted mice in vivo (24, 25, 61). The mechanisms that mediate MYXV tropism in human cancer cells are still being investigated, but one signaling requirement has been linked to the state of cellular Akt kinase activity (57). Human cancer cells (called type I) that exhibit high levels of endogenous phosphorylated Akt (Ser473 and Thr308) supported permissive MYXV replication, while cells with no detectable endogenous phosphorylated Akt, which were unaffected by the virus infection, were nonpermissive (type III). A unique subset of cancer cells (type II) were found to be permissive to wild-type MYXV but did not support MYXV replication following the deletion of the viral host range factor M-T5 (vMyxT5KO). These type II cells constitutively expressed only low levels of endogenous phosphorylated Akt (mostly at Thr308), but following infection with permissive MYXV, a significant increase in Akt phosphorylation (particularly at Ser473) was observed. In stark contrast, the endogenous levels of phosphorylated Akt remained essentially unchanged when type II cells were infected with the nonpermissive M-T5 knockout virus MYXV (vMyxT5KO) (57).
The host range factor M-T5 is essential for MYXV replication in rabbit primary lymphocytes (RL-5 cells) and for virus pathogenesis in European rabbits (31). Structurally, M-T5 possesses seven ankyrin (ANK) repeats and a carboxyl-terminal PRANC (pox protein repeats of ankyrin C-terminal) motif, which closely resembles a cellular protein motif called the F-box domain (29). Interaction between M-T5 and components of the cellular SCF (Skp-cullin-F-box) ubiquitin ligase complex was shown to protect MYXV-infected cells from cell cycle arrest (19). In MYXV-infected type II human cancer cells, physical interaction between M-T5 and cellular Akt was shown to upregulate the kinase activity of Akt (57). In another study, M-T5 was shown to be functionally interchangeable with the host ANK repeat-containing protein PIKE-A, and activation of Akt by either PIKE-A or the viral M-T5 protein was sufficient to mediate MYXV permissiveness in type II human cancer cells (59). Similarly, addition of the immunosuppressant drug rapamycin was successful at rescuing vMyxT5KO replication in type II cells by upregulating Akt activation through the mTOR pathway (47). The critical role of Akt in the regulation of multiple biological processes makes Akt a central regulator of cellular signaling, and therefore, it is not surprising that many viruses have developed sophisticated strategies for manipulating the activation of Akt (9, 11).
The serine/threonine kinase Akt (also called protein kinase B [PKB]) was initially discovered as the cellular homolog of the viral oncogene (v-Akt) carried by the AKT8 retrovirus isolated from a murine T-cell lymphoma (7, 20, 46). There are three isoforms found in mammals (Akt1, -2, and -3), encoded by separate genes but sharing over 80% amino acid sequence identity. Activation of Akt is predominantly dependent upon phosphoinositide 3-kinase (PI3K), which phosphorylates phosphoinositides (PIs) at the D3 position of the inositol ring to generate PI(3,4,5)P3 (PIP3). Akt possesses an N-terminal PH (pleckstrin homology) domain that binds PIP3 to promote its translocation of the plasma membrane. Once localized at the membrane, Akt becomes phosphorylated at residue Thr308 in the activation loop by phosphoinositide-dependent kinase 1 (PDK1) and also within the carboxy terminus at residue Ser473 by mTORC2 (mammalian target of rapamycin complex 2) (2, 49, 50). Phosphorylation of both sites is necessary for full induction of Akt kinase activity. Akt is a key regulator of many important cellular functions, including cell survival, proliferation, glucose metabolism, and protein synthesis. In the majority of human cancer cells, the Akt pathway is either mutated or constitutively activated, contributing to cancer progression through both stimulation of cellular proliferation and inhibition of apoptosis (34, 55).
In this study, we screened an array of Akt inhibitor compounds that selectively manipulate the Akt signaling network at some level and report that certain Akt inhibitors significantly blocked MYXV replication in previously permissive type I and II human cancer cells. An additional set of inhibitors selectively inhibited only the replication of MYXV deleted for M-T5 and did not modify the replicative ability of the parental wild-type virus. Furthermore, the decrease in viral replication efficiency was correlated with lower levels of phosphorylated Akt at residues Thr308 and Ser473. In contrast, certain PP2A-specific phosphatase inhibitors, such as okadaic acid, promoted increased Akt kinase activation and rescued MYXV replication in type III human cancer cells that did not previously support viral replication. Finally, we demonstrate that the hemi-phosphorylation of Akt at residue Thr308 dictates physical interaction between Akt and M-T5, which ultimately leads to productive MYXV replication in type II cancer cells. These studies show that activation of the Akt signaling cascade is essential for efficient MYXV replication in human cancer cells and further demonstrate the dynamic role by which M-T5 manipulates Akt signaling to establish a cellular environment more favorable for viral replication.
MATERIALS AND METHODS
Cells and viruses.
The cell lines used in this study included human embryonic kidney 293 (HEK293) cells and the following human tumor cell lines: human osteosarcoma (HOS) (type I) cells, human renal cancer 786-0 (type II) and Caki (type I) cells, melanoma (SK-MEL-5) (type III) cells, and the MCF-7 (type III) breast cancer cell line. All cells were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (heat inactivated), 100 U penicillin/ml, and 100 μg/ml streptomycin at 37°C in 5% CO2. The recombinant MYXVs (vMyx, strain Lausanne) used in this study included vMyx-lac (35) and vMyx-gfp (19), both of which are control viruses expressing wild-type M-T5. Additionally, vMyxT5KO (31), which is deleted for M-T5 and contains a LacZ marker between M11L and M12L, and vMyxT5KO-gfp (59), in which the enhanced green fluorescent protein (eGFP) cassette is inserted between M135R and M136R in the vMyxT5KO background (and thus contains both LacZ and eGFP), both of which fail to express M-T5, due to targeted disruption of both copies of the M-T5 open reading frame (ORF) (M005R/L), were used. All viruses were propagated and titrated by focus formation on baby green monkey kidney (BGMK) cells as described previously (35). For infection studies, cells were incubated with the indicated multiplicity of infection (MOI) of either virus for 1 h at 37°C, and infected cells were then washed three times with phosphate-buffered saline (PBS) to remove excess virus and cultured in normal medium until used in subsequent experiments.
Drug inhibition studies.
For each inhibitor, confluent cultures of cells in six-well plates were preincubated for 4 h at 37°C with the working concentration of the drug. The inhibitor solution was removed, and the cells were washed and infected with MYXV at an MOI of 3. After 1 h, excess virus was removed, and cells were cultured for 48 h in medium containing the specific inhibitor. Cultures infected in the absence of inhibitor served as controls. The number of green fluorescent protein (GFP)-expressing cells or foci present in wells infected with vMyx-gfp or vMyxT5KO-gfp was assayed by fluorescence microscopy, and efficiency of infection was determined by comparing the GFP levels present in untreated, infected controls to those in treated, infected cells. All inhibitors were purchased from Calbiochem and initially reconstituted according to the manufacturer's directions in either dimethyl sulfoxide (DMSO) or water. The working concentrations of the drugs were prepared by dilution in DMEM and used as follows unless stated otherwise: Akt inhibitor I at 5 μM; Akt inhibitor V, triciribine (API-2) at 10 μM; Akt inhibitor VIII, isozyme selective, Akti-1/2 at 2 μM; Akt inhibitor X at 3 μM; rapamycin at 20 nM; endothall at 90 nM; fenvalerate at 4 nM; DARPP-32, phospho, rat, recombinant, Escherichia coli at 1 μM; α-naphthyl acid phosphate, monosodium salt at 1 mM; and okadaic acid, Prorocentrum sp. at 0.1 nM. FTY720 was purchased from Clayman Chemicals, and the cytotoxic effects of the drug on the HOS, 786-0, and SK-MEL-5 cell lines were determined by using the CellTiter 96 nonradioactive cell proliferation assay (MTT) from Promega. Three independent experiments were performed to determine a 10% inhibitory concentration (IC10) dose of 6 μM, which was used as the working concentration.
Viral growth curves.
Viral replication was analyzed by single-step growth curve analysis as outlined previously (54). Briefly, HOS, Caki, 786-0, or SK-MEL-5 cells (5 × 105) were either mock treated or preincubated with drug for 4 h prior to infection with vMyx-gfp or vMyxT5KO-gfp at an MOI of 3 for 1 h. Unabsorbed virus was removed by washing the cells with serum-free medium three times, and cells were grown in complete growth medium supplemented with 10% FBS. Cells were harvested following infection at the indicated time points, and virus titers were determined by serial dilution and infection of BGMK cells. All growth analyses were performed in triplicate, and data were expressed as numbers of FFU per 106 cells.
Plasmids.
The plasmid 1036 pcDNA3 Myr HA Akt1 (HA-Akt), which has a N-terminal myristoylation sequence followed by the hemagglutinin (HA) epitope, was acquired from Addgene and previously documented (39). Another plasmid, 1014 pcDNA3 T7 Akt1 K179M T308A S473A (39), was also purchased from Addgene and was subsequently subcloned into the vector pcDNA3/myr-HA (HA-AktΔ3). Alternatively, HA-AktT308A, HA-AktK179M, and HA-AktΔ2 were generated by amplifying the right and left flanks at the point of mutation, using the primer sets listed in Table 1. Afterwards, a second round of PCR was performed using the forward primer Akt.for (5′-GGATCCATGAGCGACGTGGCTATTGTGAAGGA-3′) (BamHI underlined) and the reverse primer Akt.rev (5′-CTCGAGGGCCGTGCTGCTGGCCGAGTA-3′) or Akt.S473A.rev (5′-CTCGAGGGCCGTGCTGCTGGCCGAGTAGGCGAACTGGGGGAAGT-3′) (EcoRI underlined), whereas HA-AktS473A was cloned using the primer set comprising Akt.for and Akt.S473A.rev. PCR products were digested with BamHI and EcoRI, gel purified (Qiagen), and cloned directly into the vector pcDNA3/myr-HA. The identities of all clones were confirmed by sequence analysis, and expression of fusion proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. The other plasmids used in this study were MT5/myc-His and MT5-GST.
TABLE 1.
Primers used to PCR amplify Akt constructs
| Primer | Sequence |
|---|---|
| Akt.for | GGATCCATGAGCGACGTGGCTATTGTGAAGGA |
| Akt.rev | CTCGAGGGCCGTGCTGCTGGCCGAGTA |
| Akt.T308A.for | GGATCCAAGATCGCAGACTTCGGGCT |
| Akt.T308A.rev | TCGAGCGAAGTCTGCGATCTTAATGTGC |
| Akt.S473A.rev | TCGAGGGCCGTGCTGCTGGCCGAGTAGGCGAACTGGGGGAAGT |
| Akt.K179 M.for | GGATCCCTACGCCATGATGATCCTCAA |
| Akt.K179 M.rev | CTCGAGTTGAGGATCATCATGGCGTAG |
Immunoblot analysis.
Samples were fractionated by SDS-PAGE. Separated proteins were transferred to nitrocellulose and blocked with 5% skim milk in PBST (PBS and 0.1% Tween 20). Primary antibodies were diluted in 5% skim milk-PBST and incubated with membranes overnight at 4°C. Membranes were washed and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies diluted 1:5,000 in 5% milk-PBST. Immunoreactive proteins were detected by chemiluminescence (PerkinElmer). The polyclonal antibodies used were those for detecting human Akt, phospho-Akt (Thr308), and phospho-Akt (Ser473) (Cell Signaling) and glutathione S-transferase (GST) (NeoMarkers). The monoclonal antibody specific for the HA epitope (12CA5; Roche) was also used. Horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies were obtained from Jackson ImmunoResearch Laboratories.
GST pulldown assay.
For GST fusion proteins, plasmids were expressed by in vitro transcription-translation (TNT) according to the manufacturer's protocol (Promega) and were incubated with glutathione agarose beads for 2 h. Beads were pelleted by centrifugation and washed 5 times, and the immunocomplexes were resolved by SDS-PAGE. Immunoblot analyses were performed as described above, using the appropriate antibodies.
AlphaScreen protein interaction protocol.
Cells were seeded in six-well plates at a density of 5 × 105 cells per well in complete growth medium with 10% FBS. Transfections were performed with Effectene (Qiagen) in accordance with the manufacturer's instructions. Cultured cells were collected and cell lysis was prepared as previously described. All AlphaScreen assays described were performed in triplicate with 384-well white opaque plates (PerkinElmer) by using PBS containing 0.1% BSA as a buffer. For detection of HA fusion proteins, an HA detection kit containing anti-HA-coated acceptor beads (PerkinElmer) was used. Equal concentrations of acceptor beads and streptavidin donor beads were used at a final concentration of 20 μg/ml in a final volume of 25 μl per well. First, 5 μl of cell lysate, followed by biotinylated-anti-HIS (10 nM) or biotinylated-anti-Flag antibody (25 nM) and acceptor beads in buffer, was added to each well and incubated for 2 h at room temperature. A total of 5 μl of a 1:50 dilution of the donor beads was then added to give a final volume of 25 μl, and the mixture was incubated at room temperature for 2 h. All additions and incubations were made in subdued lighting conditions due to the photosensitivity of the beads, and finally, the assay plates were read with an EnVision plate reader (PerkinElmer). Likewise, AlphaScreen SureFire phospho-Akt (Thr308) and phospho-Akt (Ser473) assay kits were used according to the manufacturer's recommendations to detect Akt phosphorylation.
RESULTS
Certain Akt inhibitors block MYXV replication in permissive type I and II human cancer cells.
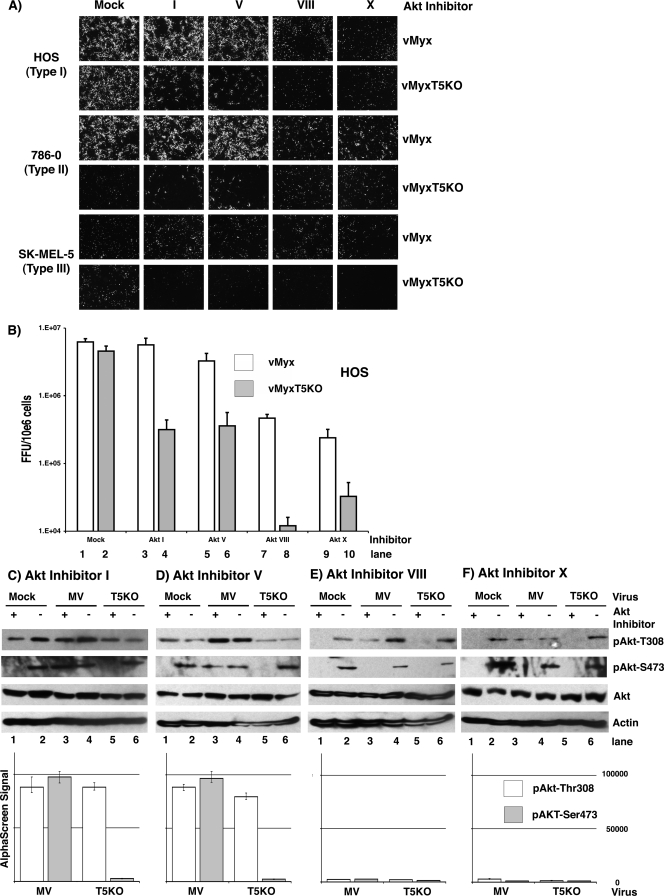
An ever-increasing number of compounds and drugs have been characterized for their ability to target various components of the PI3K-Akt signaling pathway and provide novel mechanisms for selectively manipulating the Akt network (13). To further examine the mechanism by which the phosphorylation status of Akt affects MYXV replication in human cancer cells, a collection of small molecular compounds that specifically target the Akt signaling pathway at diverse stages was examined for the ability to regulate MYXV replication in permissive and nonpermissive human cell lines. Akt inhibitor I is a D3-modified PI ester analog, a competitive inhibitor of PI3K with respect to PI (18), whereas Akt inhibitor V is a synthetic triacyclic nucleoside which selectively blocks phosphorylation and activation of Akt but does not inhibit kinase activity or upstream Akt activators such as PI3K and PDK1 (62). Interaction of Akt inhibitor VIII with the PH domain of Akt prevents the conformational change required for phosphorylation by upstream kinases, thus blocking the phosphorylation of Akt (5, 23). In contrast, Akt inhibitor X is an N-substituted phenoxazine that inhibits the activity of Akt even in the absence of its PH domain, and it has been suggested that it may bind in the ATP binding site (56). Type I, II, and III human cancer cells were treated with various inhibitors (Akt Inhibitors I, V, VIII, and X) prior to infection with either vMyx-gfp or vMyxT5KO-gfp at an MOI of 3, and viral replication was determined by the formation of green fluorescent protein-expressing foci as viewed by fluorescence microscopy at 48 hours postinfection (hpi). In HOS (type I) cells, Akt inhibitors VIII and X were able to successfully reduce viral replication of both vMyx-gfp and vMyxT5KO-gfp, while Akt inhibitors I and V were able to reduce replication of vMyxT5KO-gfp only (Fig. 1A, upper two panels). As demonstrated previously, 786-0 (type II) cells do not support replication of vMyxT5KO, whereas the wild-type virus grows permissively (57). In the presence of Akt inhibitors VIII and X, these cells became less permissive to vMyx-gfp replication as well; however, Akt inhibitors I and V did not effect MYXV replication in these type II cells (Fig. 1A, middle two panels). SK-MEL-5 (type III) cells do not support MYXV replication, and none of the Akt inhibitors rescued MYXV replication in these cells (Fig. 1A, lower two panels).
FIG. 1.
Effects of Akt inhibitors on MYXV replication in human cancer cells. (A) HOS, 786-0, and SK-MEL-5 cells were infected with either vMyx-gfp or vMyxT5KO-gfp in the absence (mock) or presence of various Akt inhibitors, and viral foci were detected by florescence microscopy at 48 hpi. Inhibitor concentrations are as stated in Materials and Methods. (B) Permissive HOS cells were infected with either vMyx-gfp (white bars) or vMyxT5KO-gfp (gray bars) at an MOI of 1 in the in the absence (mock) or presence of various Akt inhibitors, virus was collected at 48 hpi, and virus titer was determined using BGMK cells. Titers are expressed as numbers of FFU/106 cells and represent the means ± standard deviations of results from triplicate wells. (C to F) Representative Western blots (upper panels) and results of AlphaScreen SureFire assays (lower panels) illustrate Akt phosphorylation in cell lysates collected from HOS cells mock infected (lanes 1 and 2) or infected with either vMyx (MV; lanes 3 and 4) or vMyxT5KO (lanes 5 and 6) at MOI 3 in the presence (+) or absence (−) of various Akt inhibitors. Kinase activation of Akt is demonstrated by detection of specific phosphorylated forms pAkt-Thr308 (white bars) and pAkt-Ser473 (gray bars). Equal sample loading was confirmed by detection of total Akt and the housekeeping protein actin. For AlphaScreen SureFire, each sample was assayed in triplicate, and standard deviations are represented by the error bars.
To quantitatively assess the generation of infectious progeny MYXV, HOS cells were individually treated with these Akt inhibitors and infected with either vMyx-gfp or vMyxT5KO-gfp. Samples were harvested for infectious virus at 48 hpi and titrated on BGMK cells by serial dilution. Viral titers of vMyx-gfp were considerably reduced when cells were treated with Akt inhibitors VIII and X (Fig. 1B, lanes 7 and 9 versus lane 1), whereas cells treated with Akt inhibitors I and V produced viral progeny levels comparable to those observed for control treated cells (Fig. 1B, lanes 3 and 5 versus lane 1). In cells infected with vMyxT5KO-gfp, reduced viral titers were observed in samples treated with all four Akt inhibitors (Fig. 1B, lanes 4, 6, 8, and 10 versus lane 2).
Successful MYXV replication in human cancer cells is dependent upon activation of endogenous Akt (57), suggesting that in the presence of these Akt inhibitors, a reduction in MYXV replication efficiency can be attributed to a decrease in the level of phosphorylated Akt. Thus, cell lysates of virus-infected HOS (type I) cells treated with each of these Akt inhibitors were collected, and phosphorylation of Akt at residues Thr308 and Ser473 was monitored by Western blotting using Akt phospho-specific antibodies (Fig. 1C to F, upper panels). As predicted, the levels of Akt phosphorylation at both phospho residues were dramatically reduced in the presence of Akt inhibitor VIII or X in cells infected with vMyxT5KO (Fig. 1E and F, lanes 5 versus lanes 6). Furthermore, drops in Akt phosphorylation at residue Ser473 and, to a lesser extent, at Thr308 were observed in cells treated with either Akt inhibitor VIII or Akt inhibitor X and infected with vMyx (Fig. 1E and F, lanes 3 versus lanes 4). No significant decrease in Akt phosphorylation, at either Thr308 or Ser473, was observed in cells treated with Akt inhibitor I or V and infected with vMyx (Fig. 1C and D, lanes 3 versus lanes 4). In contrast, cells treated with Akt inhibitor I or V and infected with the M-T5 knockout virus (vMyxT5KO) exhibited levels of Akt phosphorylation that were considerably reduced at residue Ser473 but not Thr308 (Fig. 1C and D, lanes 5 versus lanes 6). Elevated levels of endogenous Akt phosphorylation at both the Thr308 and the Ser473 residues were observed in uninfected cells in the absence of any Akt inhibitor (Fig. 1C to F, lane 1). Conversely, all Akt inhibitors were successful at reducing the level of phosphorylated Akt at residue Ser473 in uninfected cells, but only Akt inhibitors VIII and X also blocked phosphorylation of Thr308 (Fig. 1C to F, lanes 1 versus lanes 2).
To complement the Western blot results, the levels of phosphorylated Akt were also assayed by the SureFire modification of AlphaScreen (Fig. 1C to F, lower panels). SureFire is a homogenous-bead-based platform that uses AlphaScreen technology to provide an extremely sensitive and rapid assay for quantitating the site and extent of Akt phosphorylation. Like all proximity-based assays, AlphaScreen depends on the bringing together of two distinct types of beads whose proximity causes the production of an emission fluorescence signal. The SureFire assay employs phospho and nonphospho antibodies, specific to Akt, which coat the surfaces of the beads. Only phosphorylated Akt interacts with both antibodies and brings the two sets of beads together, thus producing an emission signal that is detected. As demonstrated, the relative levels of endogenous phosphorylated Akt at residues Thr308 (Fig. 1C to F, white bars) and Ser473 (gray bars) were decreased in cells treated with Akt inhibitor VIII or X and then infected with either virus (Fig. 1E and F). Alternatively, Akt phosphorylation levels in cells treated with Akt inhibitor I or V and infected with vMyx remain high (Fig. 1C and D). Furthermore, decreased levels of phosphorylated Akt at residue Ser473, but not Thr308, were observed in vMyxT5KO-infected cells treated with Akt inhibitor I or V (Fig. 1C and D). Since we cannot create a standard curve for the AlphaScreen assay with respect to increasingly phosphorylated Akt, we are unable to propose a correlation between the intensity of the AlphaScreen signal and the molar level of Akt phosphorylation. It is our contention that the AlphaScreen technology can detect lower levels of phosphorylated Akt than the Western blots because even transient linking of the donor and acceptor beads will generate an emission signal. On the basis of the data that we present, we conclude that when cells express elevated levels of phosphorylated Akt as measured on Western blots, they will also be found to produce an increased emission signal when assayed by AlphaScreen, but not necessarily vice versa. Lastly, drug treatment or virus infection did not affect the total amount of Akt protein present in the cell (Fig. 1C to F, upper panels). Taken together, these data demonstrate that manipulation of the Akt signaling network by small molecular inhibitors can have profound effects on MYXV tropism in human cancer cells. Specifically, two of these inhibitors (I and V) can distinguish between infections with wild-type MYXV and vMyxT5KO in type I and II cells, and their inhibitory effects are suppressed in the presence of M-T5.
Dephosphorylation of Akt by FTY720 blocks MYXV replication.
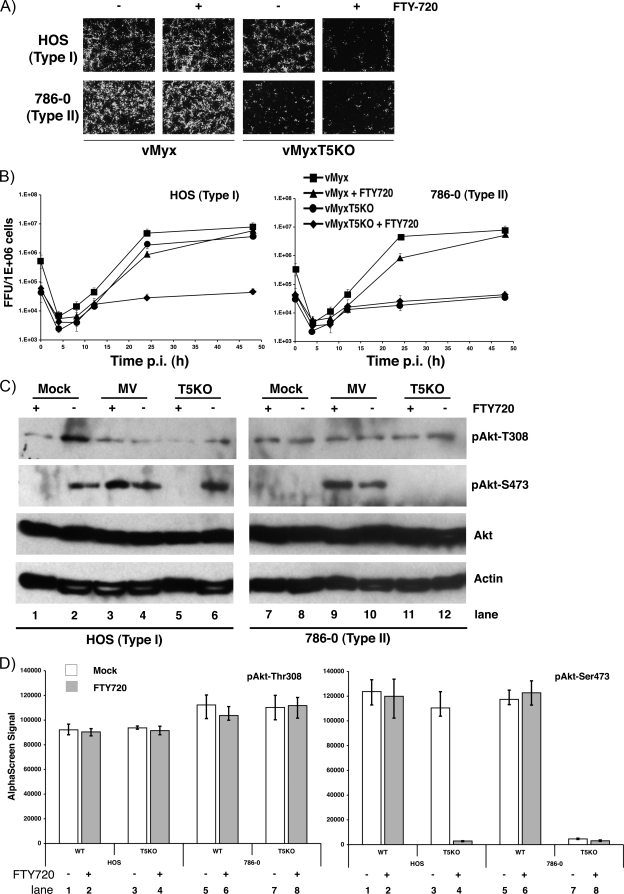
The immunosuppressant FTY720 is a derivative of ISP-1 (myriocin) and was originally demonstrated to prolong allograft transplant survival in numerous models by inhibiting lymphocyte emigration from lymphoid organs (32, 53). FTY720 induces G0/G1 arrest in Jurkat and HL-60RG cells via dephosphorylation of retinoblastoma protein (33) and promotes apoptosis in the human prostate cell line DU145 (43, 58). Furthermore, FTY720 was shown to dephosphorylate Akt, resulting in the enhancement of apoptosis via the mitochondrial pathway (22, 26). To examine if FTY720 could inhibit MYXV replication via Akt dephosphorylation and whether M-T5 could circumvent this block, type I and II cells were treated with or without FTY720 prior to infection with either vMyx-gfp or vMyxT5KO-gfp. Viral replication was determined by the formation of fluorescent green foci as viewed by fluorescence microscopy. Replication of the wild-type virus in the presence of FTY720 was not affected in permissive cell lines (type I [HOS] and type II [786-0]); however, treatment of HOS cells with FTY720 considerably reduced replication of vMyxT5KO-gfp (Fig. 2A). This phenotype was not observed in the type II cells, because they already do not support replication of vMyxT5KO.
FIG. 2.
Inhibition of vMyxT5KO replication by FTY720. (A) HOS, 786-0, and SK-MEL-5 human cancer cells were infected with either vMyx-gfp or vMyxT5KO-gfp in the absence (−) or presence (+) of FTY720, and at 48 hpi, viral focus formation was determined by florescence microscopy. (B) HOS (left) and 786-0 (right) cells were infected with either vMyx-gfp or vMyxT5KO-gfp at an MOI of 1 in the in the absence or presence of FTY720, and the titer of virus collected at various hpi was determined using BGMK cells. Titers are expressed as numbers of FFU/106 cells and represent the means ± standard deviations of results from triplicate wells. (C) Representative Western blots showing detection of Akt in cell lysates from HOS (left) and 786-0 (right) cells at 24 hpi following mock infection (lanes 1 and 2), or infection with either vMyx (lanes 3 and 4) or vMyxT5KO (lanes 5 and 6) at an MOI 3 in the in the presence (+) or absence (−) of FTY720. Kinase activation of Akt is demonstrated by detection of specific phosphorylated forms pAkt-Thr308 and pAkt-Ser473. Equal sample loading was confirmed by detection of actin. (D) Cell lysates of HOS cells prepared from those shown in panel C were assayed for Akt phospho-Thr308 (left) and Akt phospho-Ser473 (right) by using the standard AlphaScreen SureFire protocol. Each sample was assayed in triplicate, and standard deviations are represented by the error bars. Mock-treated cells are represented by white bars, whereas cells treated with FTY720 are represented by gray bars. WT, wild type.
To further examine viral replication in the presence of FTY720, viral one-step growth curves were performed in type I and II human cancer cell lines. In HOS cells, both viruses replicated efficiently and to similar levels in the absence of FTY720; however, virus titers of vMyxT5KO were significantly and uniquely lower in the presence of drug (Fig. 2B, left panel). In contrast, FTY720 had little or no inhibitory effect on viral titers of HOS cells infected with vMyx (Fig. 2B, left panel). Likewise, replication kinetics of wild-type virus in the 786-0 cells were relatively similar in the presence and absence of drug (Fig. 2B, right panel). As previously demonstrated, 786-0 cells do not support replication of vMyxT5KO (Fig. 2B, right panel).
On the basis of the reported ability of FTY720 to dephosphorylate Akt, cell lysates of MYXV-infected type I and II cells were collected, and Akt phosphorylation was analyzed by both Western blot and AlphaScreen SureFire assays. The addition of FTY720 had little or no effect on the phosphorylation status of Akt at residue Thr308 in the two cell lines in the presence or absence of either vMyx or vMyxT5KO (Fig. 2C and D, left panel). Conversely, phosphorylation of Akt at residue Ser473 was significantly reduced in HOS cells following treatment with FTY720 (Fig. 2C, lane 1 versus lane 2). However, in HOS cells treated with FTY720 and infected with wild-type MYXV but not vMyxT5KO, phosphorylation of Akt at residue Ser473 remained relatively unchanged (Fig. 2C, lane 3 versus lane 4, and D, lane 1 versus lane 2). Elevated levels of Akt phosphorylation (Ser473) were observed in 786-0 cells in the presence or absence of FTY720 only when cells were infected with vMyx (Fig. 2C, lanes 9 and 10, and D, right panel, lanes 5 and 6). The data indicate that FTY720 dephosphorylates Akt at residue Ser473, which coincidently blocks vMyxT5KO replication in previously permissive HOS (type I) cells but not vMyx, which can exploit M-T5 to keep this site phosphorylated.
Phosphatase inhibitors promote MYXV replication in previously nonpermissive human cancer cells.
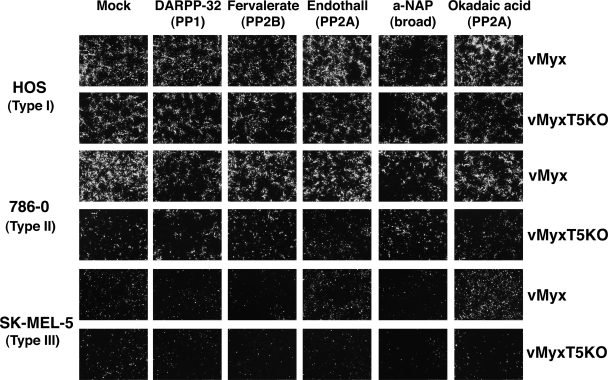
Activation of Akt is highly regulated by the intricate role of kinases and phosphatases that function to control the addition or removal of phosphates at the residues Thr308 and Ser473. Two phosphatases, protein phosphatase 2A (PP2A) and the recently identified PH domain leucine-rich repeat protein phosphatase (PHLPP), inactivate Akt via the dephosphorylation of residues Thr308 and Ser473, respectively. In nonpermissive type III human cancer cells, the level of endogenous phosphorylated Akt remains extremely low even in the presence of virus that expresses M-T5, suggesting the possibility that these phosphatases may be overexpressed or excessively active. To test this hypothesis, a collection of protein phosphatase inhibitors were selected and tested for their ability to rescue MYXV replication in nonpermissive human cancer cells. HOS, 786-0, and SK-MEL-5 cells were treated with one of the phosphatase inhibitors (DARPP-32 [PP1], endothall [PP2A], fenvalerate [PP2B], α-napthyl acid phosphate [broad spectrum], and okadaic acid [PP2A]) prior to infection with either vMyx-gfp or vMyxT5KO-gfp, and viral replication was determined by the formation of fluorescent green foci as viewed by fluorescence microscopy (Fig. 3). In the HOS cells, none of the phosphatase inhibitors appeared to significantly alter replication of either virus, as based on the production of fluorescent green foci. The phosphatase inhibitors were unsuccessful at restoring replication of vMyxT5KO in the 786-0 cells and did not change the replicative ability of vMyx. In stark contrast, the PP2A inhibitor okadaic acid was capable of releasing the replicative block in SK-MEL-5 cells to restore vMyx permissivity. Endothall, another PP2A inhibitor, had an effect similar to that observed for okadaic acid, but to a lesser extent. However, neither okadaic acid nor endothall was able to rescue replication of vMyxT5KO in the SK-MEL-5 cells. These findings suggest that inhibition of the PP2A phosphatase can restore vMyx permissivity in type III human cancer cells that previously did not support viral replication.
FIG. 3.
PP2A-specific protein phosphatases rescue MYXV replication in nonpermissive type III cancer cells. MYXV focus formation was detected at 48 hpi by florescence microscopy in HOS, 786-0, and SK-MEL-5 cells infected with either vMyx-gfp or vMyxT5KO-gfp in the absence (mock) or presence of various protein phosphatase inhibitors. a-NAP, α-naphthyl acid phosphate, monosodium salt.
The combination of okadaic acid and rapamycin induces full Akt phosphorylation to rescue vMyxT5KO replication.
To confirm the ability of okadaic acid to rescue MYXV replication in type III cells, viral titers of infected cells were determined. The viral titers of both vMyx-gfp and vMyxT5KO-gfp in HOS and 786-0 cells treated with and without okadaic acid were indistinguishable (Fig. 4A, lanes 1 to 8). In contrast, SK-MEL-5 cells treated with okadaic acid prior to infection with vMyx resulted in a significantly higher viral titer than cells not treated with okadaic acid (Fig. 4A, lane 9 versus lane 10), whereas no increase in viral titer was observed in type III cells treated with okadaic acid and infected with vMyxT5KO-gfp (Fig. 4A, lane 11 versus lane 12).
FIG. 4.
Combination of okadaic acid plus rapamycin enhances vMyxT5KO replication in type III cancer cells. The effect of okadaic acid on MYXV replication in HOS (lanes 1 to 4), 786-0 (lanes 5 to 8), and SK-MEL-5 (lanes 9 to 12) cells. All cells were either mock-treated (white bars) or preincubated with okadaic acid (gray bars) for 4 h prior to infection with vMyx-gfp (WT) or vMyxT5KO-gfp (T5KO), and at 48 hpi, focus formation was determined by florescence microscopy. (B) Single-step growth analysis of vMyx-gfp (open bars) and vMyxT5KO-gfp (gray bars) at 48 hpi in type III SK-MEL-5 cells. Prior to viral infection, cells were mock treated (lanes 1 and 2) or treated with either okadaic acid (lanes 3 and 4), rapamycin (lanes 5 and 6), or both okadaic acid and rapamycin (lanes 7 and 8). Titers are expressed as numbers of FFU/106 cells and represent the means ± standard deviations of results from triplicate wells. (C) Cell lysates were prepared from type III SK-MEL-5 cells mock infected (lanes 1 to 4) or infected with either vMyx (lanes 4 to 8) or vMyxT5KO (lanes 9 to 12) at an MOI of 3 in the absence (−) or presence (+) of okadaic acid and/or rapamycin.
Previous work in our laboratory demonstrated that the drug rapamycin was able to dramatically increase vMyxT5KO replication and spread in type II but not type III human cancer cells. Furthermore, rapamycin treatment was shown to induce Akt phosphorylation via the mTOR signaling network (47). The combination of okadaic acid and rapamycin was used to treat SK-MEL-5 cells prior to infection with either vMyx-gfp or vMyxT5KO-gfp, and viral titers were determined at 48 hpi. In the presence of okadaic acid and rapamycin, the titer of vMyxT5KO was dramatically higher than that observed in the presence of either drug alone or no drug (Fig. 4B, lane 8 versus lanes 4 and 6). Interestingly, the combination of okadaic acid and rapamycin also significantly increased wild-type vMyx replication in comparison to that observed in the presence of okadaic acid only (Fig. 4B, lane 3 versus lane 7).
The endogenous level of phosphorylated Akt in the SK-MEL-5 cells was measured, and treatment with okadaic acid and rapamycin considerably increased the phosphorylation levels at both the Thr308 and the Ser473 residues (Fig. 4C, lane 4). This pattern of Akt phosphorylation was also observed in vMyxT5KO-infected cells (Fig. 4C, lane 12). Cells infected with wild-type vMyx and treated with either okadaic acid or rapamycin expressed higher levels of Akt phosphorylation; however, the level of Akt phosphorylation was appreciably increased following treatment with the combination of drugs (Fig. 4C, lanes 6 and 7 versus lane 8). We currently do not understand why increased Akt phosphorylation was observed only in SK-MEL-5 cells treated with okadaic acid or rapamycin alone and infected with wild-type vMyx (Fig. 4C, lanes 6 and 7), not in the absence of virus (Fig. 4C, lanes 2 and 3) or in vMyxT5KO-infected cells (Fig. 4C, lanes 10 and 11). Our preferred explanation is that rapamycin-induced phosphorylation of Akt is necessary, but alone is not sufficient, to relieve the block to MYXV in type III cells. An additional, still-undefined block, downstream of Akt phosphorylation, likely exists in SK-MEL-5 cells to prevent the activation of target signaling pathways necessary to restore viral replication. However, the results clearly demonstrate that M-T5 influences the ability of both of these drugs to upregulate Akt activation in type III cancer cells. Pharmacological manipulation of the Akt signaling network may provide a mechanism by which cellular tropism can be altered, thus offering clues into the cellular blocks that inhibit MYXV replication in nonpermissive human cancer cells. The results indicate that okadaic acid and rapamycin facilitate MYXV replication in type III cancer cells by increasing Akt phosphorylation at both Thr308 and Ser473.
M-T5 binding is dependent upon prior Akt phosphorylation.
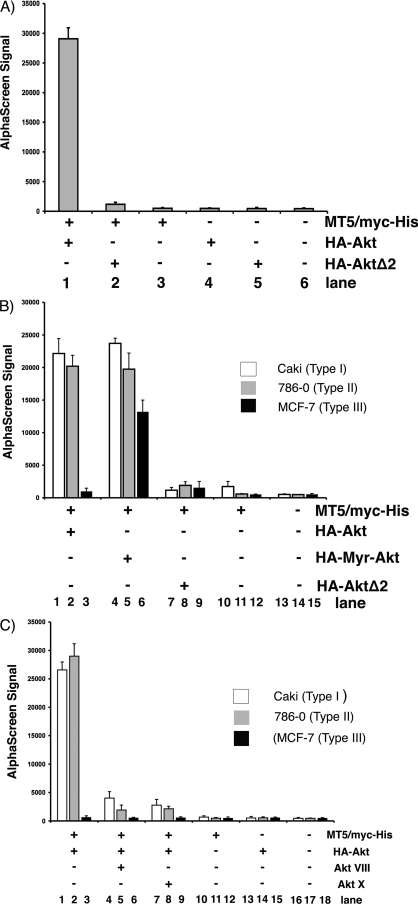
Although MYXV is a rabbit-specific poxvirus pathogen, a broad collection of human cancer cells can support productive viral replication (54). Permissive type I cancer cells were found to possess high levels of endogenously activated Akt, whereas in nonpermissive type III human cancer cells, the level of endogenous phosphorylated Akt remained essentially nondetectable, even following wild-type MYXV infection (57). To investigate whether full or partial phosphorylation of Akt might be critical for binding M-T5, HEK293 cells were cotransfected with MT5/myc-His and either HA-Akt or dominant-negative Akt (HA-AktΔ2), in which the two major sites of ligand-induced phosphorylation (Thr308 and Ser473) are both replaced by alanine. Cells transfected with M-T5 and wild-type Akt were found to produce a strong binding signal when lysates were tested for M-T5/Akt binding by AlphaScreen the following day (Fig. 5A, lane 1). Alternatively, transfection of HA-AktΔ2 dramatically reduced binding affinity between the two proteins (Fig. 5B, lane 2), suggesting that at least some phosphorylation of Akt is critical for M-T5 binding.
FIG. 5.
The cellular environment regulates the interaction between Akt and M-T5. Cells were transiently transfected with the specified (+) combination of plasmids (MT5/myc-His, HA-Akt, HA-AktΔ2, and HA-Myr-Akt) in HEK293 (A) or Caki, 786-0, and MCF-7 (B and C) cell lines. Cell lysates were harvested after 48 h and preincubated with biotin conjugated anti-Myc antibody and donor/acceptor beads before an AlphaScreen assay was performed to determine binding. Each sample was assayed in triplicate, and the error bars represent standard deviations. (C) Cells were either mock treated or treated with Akt inhibitor VIII or X prior to collection of cell lysis and AlphaScreen analysis of M-T5 binding to Akt.
Subsequently, the importance of Akt phosphorylation for binding M-T5 was examined using human cancer cells. Type I (Caki), II (786-0), and III (MCF-7) human cancer cells were cotransfected with MT5/myc-His and either HA-Akt, a constitutively active mutant of Akt (HA-Myr-Akt), or HA-AktΔ2. The kinase activity of Myr-Akt, which consists of Akt1 fused to a myristoylation sequence, is much greater than that of the wild-type Akt enzyme. A distinct M-T5/Akt binding signal was observed in both type I and type II cells cotransfected with HA-Akt and MT5/myc-His (Fig. 5B, lanes 1 and 2); however, in type III cells, only a relatively low-level binding signal was detected (Fig. 5B, lane 3), suggesting that M-T5 was essentially unable to bind Akt in these MYXV-nonpermissive cells. Strikingly, transfection of HA-Myr-Akt promoted binding to MT5/myc-His in type III human cancer cells, as demonstrated by the increase in binding signal (Fig. 5B, lane 6). In contrast, binding between Akt and M-T5 was completely lost in type I, II, and III human cancer cells when MT5/myc-His was cotransfected with HA-AktΔ2 (Fig. 5B, lanes 7 to 9). Our findings suggest that at least some phosphorylation of Akt is critical for binding M-T5 in both HEK293 and human cancer cells and that manipulation of the Akt phosphorylation status can perturb the interaction between these two binding partners.
Since some phosphorylation of Akt is critical for physical binding to M-T5, we next investigated the ability of Akt inhibitors VIII and X to block the interaction of these binding partners. Type I, II, and III human cancer cells were cotransfected with MT5/myc-His and HA-Akt and were treated with either Akt inhibitor VIII or Akt inhibitor X the following day. Cell lysates were collected 2 days after transfection and were assayed for M-T5/Akt binding by AlphaScreen. In the presence of either inhibitor, the binding affinity between M-T5 and Akt, as demonstrated by the signal strength, was significantly reduced in type I (Fig. 5C, compare lane 1 to lane 4 or 7) and type II (Fig. 5C, compare lane 2 to lane 5 or 8) human cancer cells. No interaction between M-T5 and Akt in the type III cells was observed in the presence or absence of inhibitors (Fig. 5C, lanes 3, 6, and 9), in agreement with the results shown in Fig. 5B. Taken together, these data provide evidence for the molecular interaction between M-T5 and Akt and the importance of at least some Akt phosphorylation prior to M-T5 binding.
Phosphorylation of Akt residue Thr308 promotes M-T5 binding.
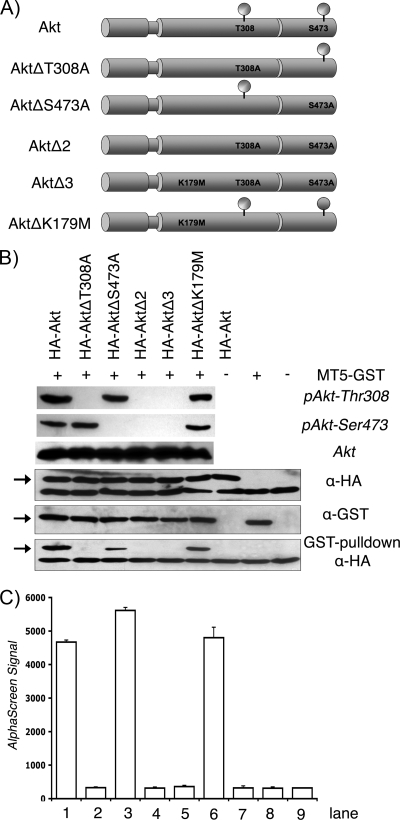
To further examine how Akt phosphorylation dictates M-T5 binding, a collection of Akt mutants was constructed (Fig. 6A) and assayed for interactions by a GST pulldown assay and AlphaScreen. Each plasmid was sequence verified, and Akt protein expression and phosphorylation were confirmed by Western blot analysis using phospho- and non-phospho-specific Akt antibodies (Fig. 6B, upper three panels). A coupled in vitro transcription-translation (TNT) protocol was used to coexpress MT5-GST, tagged at the C terminus with GST, plus one of the Akt proteins fused to a common N-terminal HA tag (HA-Akt, HA-AktΔT308A, HA-AktΔS473A, HA-AktΔ2, HA-AktΔ3, and HA-AktΔK179M). Samples were then incubated with GST-coated beads to pull down MT5-GST fusions, and complexes were resolved by SDS-PAGE and immunoblotted with anti-HA antibody to analyze binding of HA-tagged Akt proteins. Coprecipitation of MT5-GST and HA-Akt was observed only when threonine was present at Akt residue 308, whereas binding was abolished following substitution of alanine at this position (Fig. 6B, lower panels, 2nd, 4th, and 5th lanes). In contrast, mutations at either residue 473 (S → A) or residue 179 (K → M) did not significantly affect the ability of M-T5 to bind Akt (Fig. 6B, lower panels, 3rd and 6th lanes). Similar results were observed when binding between M-T5 and the various Akt mutants was assayed by AlphaScreen (Fig. 6C). These findings demonstrate that Akt phosphorylation of Thr308, but not residue Ser473, is critical for binding M-T5.
FIG. 6.
Phospho status of Akt residue Thr308 mediates M-T5 binding. (A) Schematic representation of the Akt constructs used during this study. Amino acid substitutions are indicated. All Akt constructs contain an amino-terminal HA tag. (B) The plasmid MT5-GST and the various HA-Akt constructs were coexpressed by in vitro-coupled transcription-translation and subjected to a GST pulldown assay. Precipitates and total lysates were resolved by SDS-PAGE and probed with anti-HA (α-HA) antibody to detect coprecipitated viral proteins (lower panels). Expression and phosphorylation of Akt proteins were confirmed by immunoblotting with phospho- and non-phospho-specific Akt antibodies and antibody against HA epitope (upper three panels). Furthermore, M-T5 expression was detected by an anti-GST-specific antibody. Bands of interest are represented by arrows. (C) Cell lysates were collected from transiently transfected HEK293 cells and assayed for protein-protein binding by AlphaScreen as described in the legend to Fig. 5.
DISCUSSION
An emerging body of evidence clearly demonstrates the remarkable ability of poxviruses to specifically manipulate a wide spectrum of cellular signaling networks to establish a cellular environment more favorable to viral replication (28). The PI3K-Akt-mTOR pathway has been reported to influence a diverse range of biological functions (9) and therefore provides an ideal target for poxviruses to reconfigure intracellular signaling according to their replicative requirements. The orthopoxviruses, vaccinia virus, and cowpox virus have been shown to rapidly activate the PI3K-Akt pathway following infection, and blockage of PI3K signaling with the inhibitor LY294002 significantly decreased viral titer and induced an apoptotic response (45). Similarly, MYXV tropism is dependent upon activation of Akt; however, in contrast to what was found for the orthopoxviruses, like vaccinia and cowpox virus, inhibition of PI3K by LY294002 did not block MYXV replication (57), suggesting that MYXV possesses a unique ability to modulate Akt activation directly at the level of Akt itself. On the other hand, direct inhibition of Akt phosphorylation by the transfection of a dominant-negative form of Akt significantly reduced the replicative potential of MYXV in permissive human cancer cell lines (57). In the current study, four inhibitors that function to block Akt signaling, each with a unique mode of action, were exploited to investigate the details of M-T5 interaction with Akt.
Many commercially available Akt inhibitors act by either preventing the generation of PIP3 by PI3K or blocking the binding of PIP3 to Akt. This inhibitor mode is utilized by PI analogs, such as Akt inhibitor I, which bind to the PH domain of Akt to occupy the binding site used by PIP3 (18). Another mode of inhibition involves preventing the activation of Akt via inhibition of upstream effectors. For example, the triacyclic nucleoside Akt inhibitor V targets an Akt effector molecule other than PI3K or PDK1 to selectively inhibit phosphorylation and activation of Akt (21, 62). Furthermore, this inhibitor exhibits little effect toward cellular signaling pathways mediated by PKC, PKA, SGK, Stat3, p38, extracellular signal-regulated kinase 1/2 (ERK1/2), or Jun N-terminal protein kinase (JNK) and has been shown to preferentially induce apoptosis and growth arrest in cancer cells with aberrant Akt activity both in vitro and in vivo (21, 62). Alternatively, other drugs, like Akt inhibitor VIII, specifically bind the PH domain of Akt to promote formation of an inactive conformation, which does not allow phosphorylation by upstream kinases (5, 12, 23, 64). Interestingly, Akt inhibitor VIII does not exhibit any inhibitory effects against PH domain-lacking Akt variants or other closely related AGC family kinases (PKA, PKC, or SGK), even at concentrations as high as 50 μM (5), whereas Akt inhibitor X binds in the ATP binding site of Akt and the mode of inhibition is not PH domain dependent. Subsequently, the drug inhibits insulin-like growth factor I (IGF-I)-stimulated nuclear translocation of Akt and blocks phosphorylation of the downstream Akt targets, mTOR and p70S6 kinase (56).
We report here that only Akt inhibitors VIII and X were able to reduce viral replication when cells were infected with MYXV (with or without M-T5); however, in the absence of M-T5, the level of replication of the knockout virus (vMyxT5KO) was selectively lower in type I cancer cells treated with the Akt inhibitors I and V (Fig. 1). Interestingly, neither Akt inhibitor I nor Akt inhibitor V directly targets Akt; rather, these inhibitors function by targeting elements immediately upstream of Akt itself, which may explain the inability of these inhibitors to efficiently reduce replication when M-T5 is expressed by MYXV. In other words, M-T5 can specifically circumvent the effects of these two Akt inhibitors by binding Akt directly. In contrast, Akt inhibitors VIII and X appear to block activation of Akt in a manner that M-T5 is unable to overcome, and this suggests that these two drugs block required signaling elements that are exploited when M-T5 activates Akt.
The immunomodulator FTY720 is a synthetic sphingosine analogue of myriocine and has been extensively studied in experimental allotransplantation models and autoimmune disease models (8, 10, 27, 51-53). An increasing number of studies have examined the antitumoral properties of FTY720, and in bladder cancer, breast cancer, and leukemia, FTY720 was found to be effective at inducing apoptosis (3, 4, 26). At the molecular level, FTY720 was shown to dephosphorylate Akt, resulting in enhancement of apoptosis through mitochondria by inhibition of Bcl-2 (26). Dephosphorylation of Akt at residue Ser473 was more pronounced than phosphorylation of Thr308 in both in vitro and in vivo models (22), and this specificity prompted us to examine the effects of this drug on MYXV replication. As we report here, FTY720 had little if any effect on wild-type MYXV replication; however, in the absence of M-T5, viral titers were specifically and dramatically reduced in type I human cancer cells compared to the titers observed in mock-treated cells (Fig. 2A and B). A correlation between lower viral titers of vMyxT5KO and decreased phosphorylation of Akt at residue Ser473 was observed in cells treated with FTY720. In stark contrast, no decrease in phosphorylated Akt was observed in wild-type MYXV-infected cells treated with FTY720, suggesting that M-T5 is able to counteract the mechanism by which FTY720 induces dephosphorylation of Akt in these cells (Fig. 2C and D). Similarly, reduced vMyxT5KO replication was observed in cells treated with either Akt inhibitor I or Akt inhibitor V (Fig. 1); however, the mechanisms by which these compounds block vMyxT5KO may or may not be identical. In either case, FT720 provides an alternative reagent for studying how Akt phosphorylation at specific sites influences MYXV infection in human cancer cells.
Reversible phosphorylation is an important intracellular regulatory mechanism for many diverse cellular processes. The two phosphatases PP2A and PHLPP tightly regulate the cellular activity of Akt via dephosphorylation of residues Thr308 and Ser473, respectively. Interestingly, when a collection of phosphatase inhibitors was screened for the ability to rescue MYXV replication in nonpermissive type III human cancer cells, only okadaic acid and endothall were found to be effective at rescuing virus replication. Both okadaic acid and endothall are specific inhibitors of PP2A, suggesting that inhibition of the protein phosphatase PP2A can relieve the MYXV replicative block in these cells. Furthermore, increased MYXV replication was correlated with elevated levels of Akt phosphorylation at both Thr308 and Ser473. However, in the absence of M-T5, neither okadaic acid nor endothall could successfully restore MYXV permissivity, even though increased levels of Akt phosphorylation of Akt at residue Thr308 but not Ser473 were observed. This would imply that M-T5 functions to proactively promote phosphorylation of Akt at residue Ser473, and this is necessary for productive MYXV replication to occur. Thus, the failure of MYXV to replicate in these nonpermissive type III cancer cells may be attributed to overexpression of PP2A; however, this has not been examined in further detail. In this study, the concentration of inhibitors used was low to reduce toxicity, so some of the phosphatase inhibitors that did not significantly alter MYXV replication may have been insufficiently active in these cells at the chosen concentrations.
The mTOR signaling pathway plays a critical role in cell growth, proliferation, and survival, in part by regulation of translation initiation (16). mTOR, the central component, exists in two structurally and functionally distinct protein complexes, mTORC1 and mTORC2. Upon activation, the raptor-mTOR protein complex (mTORC1) functions to increase mRNA translation via activation of p70S6 kinase and inhibition of the eIF4E-binding protein (17). Rapamycin forms a complex with FKBP12 that binds mTORC1 to specifically inhibit mTORC1 signaling (37). The rictor-mTOR protein complex (mTORC2) is largely rapamycin insensitive, was identified as the previously elusive kinase (PDK2) responsible for the phosphorylation of Akt at residue Ser473, and is required for the full activation of Akt (41). Originally characterized for its ability to block activation of mTOR, rapamycin and its analogs (rapalogs) have proven effective as anticancer agents in a broad range of preclinical models (30, 38). Recent reports have shown that prolonged inhibition of mTOR by rapamycin blocked mTORC2 assembly and subsequent activation of Akt (40, 63). Alternatively, inhibition of mTOR in some cells was demonstrated to induce Akt activation via elevated expression of the receptor tyrosine kinase insulin receptor substrate-1 (36, 44). Furthermore, our laboratory previously reported that rapamycin increased the levels of constitutively activated Akt in human giloma cells and enhanced the oncolytic potential of MYXV in an orthotopic human medulloblastoma xenograft mouse model (25). On the basis of the ability of rapamycin to promote Akt phosphorylation and rescue replication of vMyxT5KO in type II human cancer cells (47), we were prompted to investigate whether the combination of rapamycin plus okadaic acid could successfully restore vMyxT5KO replication in type III SK-MEL-5 cells (Fig. 4). Even in the absence of MYXV infection, increased Akt phosphorylation of Akt at both the Thr308 and the Ser473 residues was observed following the dual drug treatment of these cells but not following treatment with either drug alone. The results suggest that following hemi-phosphorylation of Akt at residue Thr308, M-T5 is able to promote the subsequent phosphorylation of residue Ser473 and thus fully activate Akt. Moreover, these results show that MYXV replication in type III human cancer cells can be achieved through manipulation of the PI3K-Akt-mTOR signaling cascade, which ultimately leads to the full activation of Akt.
In the majority of human cancer cells, the Akt pathway is either mutated or constitutively activated, often providing the appropriate intracellular signaling environment for a productive MYXV replication to occur. However, in type III cells that have little or no endogenous phosphorylated Akt and do not support MYXV replication, drugs that promote activation of Akt, such as okadaic acid, can be used to expand the tropism of MYXV. Obviously, this needs to be balanced by the fact that constitutive enhancement of Akt kinase activity also blocks apoptosis and promotes cellular proliferation, both of which might accelerate cancer progression in the absence of an oncolytic virus. Over 100 unique Akt kinase substrates have been identified to date, covering a broad range of cellular functions, and currently, it is not known which downstream pathway(s) contributes to the promotion of MYXV replication in human cancer cells. Thus, identification of drugs that specifically enhance MYXV replication but do not significantly influence other cellular pathways would be ideal. We have previously shown that overexpression of the cellular ANK repeat-containing protein PIKE-A is able to upregulate Akt kinase activity and promote MYXV replication in previously nonpermissive cells (59). PIKE-A, a physiological regulator of Akt, binds directly to activated Akt in a guanine nucleotide-dependent manner and stimulates the kinase activity of Akt (1). In fact, overexpression of PIKE-A in human cancer cells inhibits apoptosis by enhancing the kinase activity of Akt (1). Future studies will examine small molecular libraries for novel compounds that have the capacity to expand MYXV tropism and enhance viral replication in type III cancer cells. Furthermore, we plan to test small interfering RNA (siRNA) knockdown of specific components of the Akt signaling cascade to deconstruct the downstream pathway requirements for MYXV tropism in cancer cells. This knowledge may have significant implications for the rational design of the next generation of oncolytic viruses as the development of new and improved synergistic cancer therapies continues.
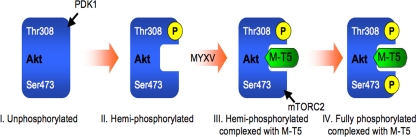
Although many virus-encoded proteins have been reported to target the PI3K-Akt-mTOR signaling cascade, M-T5 is the only viral protein shown to date that directly binds and activates Akt. Interestingly, no physical interaction between M-T5 and Akt was observed in type III cells that exhibit very low levels of constitutively phosphorylated Akt, which suggests that the phospho status of Akt may dictate the initial binding of M-T5. In support of this, when a constitutively active variant of Akt was tested, binding of M-T5 and Akt could be readily detected in each of the three classes of cancer cells. However, a nonphosphorylatable Akt variant (with alanine at the two phosphorylation sites) was unable to bind M-T5 in all three cell lines examined (Fig. 5). These results are congruent with previous studies in which expression of constitutively active Akt was able to rescue MYXV infectivity in type III human cancer cells; however, even transfection of M-T5 could not rescue MYXV replication in these cells (57), which we now believe is because M-T5 cannot bind completely unphosphorylated Akt. The rate-limiting step to Akt activation is the binding of PIP3 to the PH domain of Akt, which promotes Akt localization to the plasma membrane. Once correctly positioned at the plasma membrane, Akt can then be phosphorylated by its activating kinases, leading to full activation. Furthermore, phosphorylation of both residues is required for full Akt activity, and phosphorylation of Thr308 is not dependent on phosphorylation of Ser473 or vice versa, as measured by in vitro kinase assays (2). Analysis of Akt mutants in which the Thr308 or Ser473 sites were individually converted to alanine showed that phosphorylation of Thr308, but not residue Ser473, was critical for binding M-T5 (Fig. 6). These results suggest that MYXV tropism in human cancer is largely dependent upon the endogenous phospho status of Akt residue Thr308. As one model, phosphorylation of Akt at residue Thr308 may induce a conformational change allowing M-T5 access to a previously blocked binding site. Once M-T5 is bound to hemi-phosphorylated Akt, it is not yet known whether M-T5 alters the availability of Akt to kinases or phosphatases that regulate the Ser473 site; however, the subsequent phosphorylation of Ser473 is particularly dramatic (Fig. 7). Future studies will examine the possible mechanism(s) by which M-T5 regulates Ser473 phosphorylation, for example, by increasing access to the kinase mTORC2 or decreasing access to the protein phosphatase PHLPP.
FIG. 7.
Binding of M-T5 to cellular Akt induces phosphorylation of Akt and is a key restriction determinant for MYXV permissiveness in type II human cancer cells. Phosphorylation of Akt at residue Thr308 by PDK1 induces a conformational change to Akt, which allows M-T5 to bind. Binding of M-T5 to the hemi-phosphorylated Akt promotes the subsequent phosphorylation of Ser473 by mTORC2. Once fully phosphorylated, Akt activates various downstream signaling cascades important for MYXV replication in the human cancer cells. Type I cells are constitutively in conformation IV and are permissive for both wild-type MYXV and vMyxT5KO. Type III cells are constitutively trapped in conformation I and are nonpermissive for both wild-type MYXV and vMyxT5KO.
In summary, poxviruses encode a myriad of proteins that coordinate remarkable intracellular signaling modifications to establish an environment which will support productive virus replication. Although M-T5 has been shown to enhance MYXV replication through interaction with Akt, many of the fundamental questions regarding the mechanism of this interaction remain poorly understood. The results in this study suggest that binding of M-T5 is dependent upon the endogenous phospho status of Akt residue Thr308 and that pharmacological manipulation of the Akt signaling pathway can significantly influence the outcome of MYXV replication in human cancer cells. Thus, this knowledge may have significant implications for the identification of novel compounds for use in conjunction with MYXV virotherapy to increase the oncolytic potential of this virus. Furthermore, understanding the interaction between viral host range factors like M-T5 and cellular targets such as Akt will provide invaluable insights into how vital cellular networks can be reprogrammed by viral factors to increase virus survival at the cellular level.
Acknowledgments
This work was funded by a startup grant to G.M. from the University of Florida College of Medicine, NIH R01 AI080607, and by grant NIH U54 AI057157 to the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB). S.J.W. received funding from the University of Florida Medical Guild Research Incentive Award.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Ahn, J. Y., R. Rong, T. G. Kroll, E. G. Van Meir, S. H. Snyder, and K. Ye. 2004. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J. Biol. Chem. 279:16441-16451. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma, H., S. Takahara, S. Horie, S. Muto, Y. Otsuki, and Y. Katsuoka. 2003. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J. Urol. 169:2372-2377. [DOI] [PubMed] [Google Scholar]
- 4.Azuma, H., S. Takahara, N. Ichimaru, J. D. Wang, Y. Itoh, Y. Otsuki, J. Morimoto, R. Fukui, M. Hoshiga, T. Ishihara, N. Nonomura, S. Suzuki, A. Okuyama, and Y. Katsuoka. 2002. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 62:1410-1419. [PubMed] [Google Scholar]
- 5.Barnett, S. F., D. Defeo-Jones, S. Fu, P. J. Hancock, K. M. Haskell, R. E. Jones, J. A. Kahana, A. M. Kral, K. Leander, L. L. Lee, J. Malinowski, E. M. McAvoy, D. D. Nahas, R. G. Robinson, and H. E. Huber. 2005. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 385:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett, J. W., L. R. Alston, F. Wang, M. M. Stanford, P. A. Gilbert, X. Gao, J. Jimenez, D. Villeneuve, P. Forsyth, and G. McFadden. 2007. Identification of host range mutants of myxoma virus with altered oncolytic potential in human glioma cells. J. Neurovirol. 13:549-560. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann, V., J. G. Cyster, and T. Hla. 2004. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 4:1019-1025. [DOI] [PubMed] [Google Scholar]
- 9.Buchkovich, N. J., Y. Yu, C. A. Zampieri, and J. C. Alwine. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat. Rev. Microbiol. 6:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba, K., Y. Hoshino, C. Suzuki, Y. Masubuchi, Y. Yanagawa, M. Ohtsuki, S. Sasaki, and T. Fujita. 1996. FTY720, a novel immunosuppressant possessing unique mechanisms. I. Prolongation of skin allograft survival and synergistic effect in combination with cyclosporine in rats. Transplant. Proc. 28:1056-1059. [PubMed] [Google Scholar]
- 11.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065-1076. [DOI] [PubMed] [Google Scholar]
- 12.DeFeo-Jones, D., S. F. Barnett, S. Fu, P. J. Hancock, K. M. Haskell, K. R. Leander, E. McAvoy, R. G. Robinson, M. E. Duggan, C. W. Lindsley, Z. Zhao, H. E. Huber, and R. E. Jones. 2005. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol. Cancer Ther. 4:271-279. [PubMed] [Google Scholar]
- 13.Engelman, J. A. 2009. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9:550-562. [DOI] [PubMed] [Google Scholar]
- 14.Fenner, F. 2000. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 24:123-133. [DOI] [PubMed] [Google Scholar]
- 15.Fenner, F. 1983. The Florey lecture, 1983. Biological control, as exemplified by smallpox eradication and myxomatosis. Proc. R. Soc. Lond. B Biol. Sci. 218:259-285. [DOI] [PubMed] [Google Scholar]
- 16.Gingras, A. C., B. Raught, and N. Sonenberg. 2004. mTOR signaling to translation. Curr. Top. Microbiol. Immunol. 279:169-197. [DOI] [PubMed] [Google Scholar]
- 17.Hara, K., Y. Maruki, X. Long, K. Yoshino, N. Oshiro, S. Hidayat, C. Tokunaga, J. Avruch, and K. Yonezawa. 2002. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110:177-189. [DOI] [PubMed] [Google Scholar]
- 18.Hu, Y., L. Qiao, S. Wang, S. B. Rong, E. J. Meuillet, M. Berggren, A. Gallegos, G. Powis, and A. P. Kozikowski. 2000. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J. Med. Chem. 43:3045-3051. [DOI] [PubMed] [Google Scholar]
- 19.Johnston, J. B., G. Wang, J. W. Barrett, S. H. Nazarian, K. Colwill, M. Moran, and G. McFadden. 2005. Myxoma virus M-T5 protects infected cells from the stress of cell cycle arrest through its interaction with host cell cullin-1. J. Virol. 79:10750-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, P. F., T. Jakubowicz, F. J. Pitossi, F. Maurer, and B. A. Hemmings. 1991. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. U. S. A. 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karst, A. M., D. L. Dai, J. Q. Cheng, and G. Li. 2006. Role of p53 up-regulated modulator of apoptosis and phosphorylated Akt in melanoma cell growth, apoptosis, and patient survival. Cancer Res. 66:9221-9226. [DOI] [PubMed] [Google Scholar]
- 22.Lee, T. K., K. Man, J. W. Ho, C. K. Sun, K. T. Ng, X. H. Wang, Y. C. Wong, I. O. Ng, R. Xu, and S. T. Fan. 2004. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis 25:2397-2405. [DOI] [PubMed] [Google Scholar]
- 23.Lindsley, C. W., Z. Zhao, W. H. Leister, R. G. Robinson, S. F. Barnett, D. Defeo-Jones, R. E. Jones, G. D. Hartman, J. R. Huff, H. E. Huber, and M. E. Duggan. 2005. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 15:761-764. [DOI] [PubMed] [Google Scholar]
- 24.Lun, X., W. Yang, T. Alain, Z. Q. Shi, H. Muzik, J. W. Barrett, G. McFadden, J. Bell, M. G. Hamilton, D. L. Senger, and P. A. Forsyth. 2005. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 65:9982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lun, X. Q., H. Zhou, T. Alain, B. Sun, L. Wang, J. W. Barrett, M. M. Stanford, G. McFadden, J. Bell, D. L. Senger, and P. A. Forsyth. 2007. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 67:8818-8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka, Y., Y. Nagahara, M. Ikekita, and T. Shinomiya. 2003. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br. J. Pharmacol. 138:1303-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuura, M., T. Imayoshi, K. Chiba, and T. Okumoto. 2000. Effect of FTY720, a novel immunosuppressant, on adjuvant-induced arthritis in rats. Inflamm. Res. 49:404-410. [DOI] [PubMed] [Google Scholar]
- 28.McFadden, G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer, A. A., S. B. Fleming, and N. Ueda. 2005. F-box-like domains are present in most poxvirus ankyrin repeat proteins. Virus Genes 31:127-133. [DOI] [PubMed] [Google Scholar]
- 30.Meric-Bernstam, F., and A. M. Gonzalez-Angulo. 2009. Targeting the mTOR signaling network for cancer therapy. J. Clin. Oncol. 27:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagahara, Y., M. Ikekita, and T. Shinomiya. 2000. Immunosuppressant FTY720 induces apoptosis by direct induction of permeability transition and release of cytochrome c from mitochondria. J. Immunol. 165:3250-3259. [DOI] [PubMed] [Google Scholar]
- 33.Nagahara, Y., Y. Matsuoka, K. Saito, M. Ikekita, S. Higuchi, and T. Shinomiya. 2001. Coordinate involvement of cell cycle arrest and apoptosis strengthen the effect of FTY720. Jpn. J. Cancer Res. 92:680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 35.Opgenorth, A., K. Graham, N. Nation, D. Strayer, and G. McFadden. 1992. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 66:4720-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly, K. E., F. Rojo, Q. B. She, D. Solit, G. B. Mills, D. Smith, H. Lane, F. Hofmann, D. J. Hicklin, D. L. Ludwig, J. Baselga, and N. Rosen. 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshiro, N., K. Yoshino, S. Hidayat, C. Tokunaga, K. Hara, S. Eguchi, J. Avruch, and K. Yonezawa. 2004. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9:359-366. [DOI] [PubMed] [Google Scholar]
- 38.Plas, D. R., and G. Thomas. 2009. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr. Opin. Cell Biol. 21:230-236. [DOI] [PubMed] [Google Scholar]
- 39.Ramaswamy, S., N. Nakamura, F. Vazquez, D. B. Batt, S. Perera, T. M. Roberts, and W. R. Sellers. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. U. S. A. 96:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarbassov, D. D., S. M. Ali, S. Sengupta, J. H. Sheen, P. P. Hsu, A. F. Bagley, A. L. Markhard, and D. M. Sabatini. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22:159-168. [DOI] [PubMed] [Google Scholar]
- 41.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 42.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 43.Shen, Y., M. Cai, W. Xia, J. Liu, Q. Zhang, H. Xie, C. Wang, X. Wang, and S. Zheng. 2007. FTY720, a synthetic compound from Isaria sinclairii, inhibits proliferation and induces apoptosis in pancreatic cancer cells. Cancer Lett. 254:288-297. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y., H. Yan, P. Frost, J. Gera, and A. Lichtenstein. 2005. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol. Cancer Ther. 4:1533-1540. [DOI] [PubMed] [Google Scholar]
- 45.Soares, J. A., F. G. Leite, L. G. Andrade, A. A. Torres, L. P. De Souza, L. S. Barcelos, M. M. Teixeira, P. C. Ferreira, E. G. Kroon, T. Souto-Padron, and C. A. Bonjardim. 2009. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infectionis required for both host survival and viral replication. J. Virol. 83:6883-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staal, S. P., J. W. Hartley, and W. P. Rowe. 1977. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc. Natl. Acad. Sci. U. S. A. 74:3065-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanford, M. M., J. W. Barrett, S. H. Nazarian, S. Werden, and G. McFadden. 2007. Oncolytic virotherapy synergism with signaling inhibitors: rapamycin increases myxoma virus tropism for human tumor cells. J. Virol. 81:1251-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanford, M. M., S. J. Werden, and G. McFadden. 2007. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet. Res. 38:299-318. [DOI] [PubMed] [Google Scholar]
- 49.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 50.Stokoe, D., L. R. Stephens, T. Copeland, P. R. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormick, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, K., T. Kazui, A. Kawabe, X. K. Li, N. Funeshima, H. Amemiya, and S. Suzuki. 1998. Immunosuppressive effect of FTY 720 on rat pancreas allograft. Transplant. Proc. 30:3417-3418. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki, S., S. Enosawa, T. Kakefuda, H. Amemiya, Y. Hoshino, and K. Chiba. 1996. Long-term graft acceptance in allografted rats and dogs by treatment with a novel immunosuppressant, FTY720. Transplant. Proc. 28:1375-1376. [PubMed] [Google Scholar]
- 53.Suzuki, S., S. Enosawa, T. Kakefuda, T. Shinomiya, M. Amari, S. Naoe, Y. Hoshino, and K. Chiba. 1996. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation 61:200-205. [DOI] [PubMed] [Google Scholar]
- 54.Sypula, J., F. Wang, Y. Ma, J. Bell, and G. McFadden. 2004. Myxoma virus tropism in human tumour cells. Gene Ther. Mol. Biol. 8:103-114. [Google Scholar]
- 55.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thimmaiah, K. N., J. B. Easton, G. S. Germain, C. L. Morton, S. Kamath, J. K. Buolamwini, and P. J. Houghton. 2005. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J. Biol. Chem. 280:31924-31935. [DOI] [PubMed] [Google Scholar]
- 57.Wang, G., J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden. 2006. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. U. S. A. 103:4640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, J. D., S. Takahara, N. Nonomura, N. Ichimaru, K. Toki, H. Azuma, K. Matsumiya, A. Okuyama, and S. Suzuki. 1999. Early induction of apoptosis in androgen-independent prostate cancer cell line by FTY720 requires caspase-3 activation. Prostate 40:50-55. [DOI] [PubMed] [Google Scholar]
- 59.Werden, S. J., J. W. Barrett, G. Wang, M. M. Stanford, and G. McFadden. 2007. M-T5, the ankyrin repeat, host range protein of myxoma virus, activates Akt and can be functionally replaced by cellular PIKE-A. J. Virol. 81:2340-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo, Y., K. J. Kelly, M. M. Stanford, C. Galanis, Y. S. Chun, Y. Fong, and G. McFadden. 2008. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Ann. Surg. Oncol. 15:2329-2335. [DOI] [PubMed] [Google Scholar]
- 61.Wu, Y., X. Lun, H. Zhou, L. Wang, B. Sun, J. C. Bell, J. W. Barrett, G. McFadden, J. A. Biegel, D. L. Senger, and P. A. Forsyth. 2008. Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clin. Cancer Res. 14:1218-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, L., H. C. Dan, M. Sun, Q. Liu, X. M. Sun, R. I. Feldman, A. D. Hamilton, M. Polokoff, S. V. Nicosia, M. Herlyn, S. M. Sebti, and J. Q. Cheng. 2004. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 64:4394-4399. [DOI] [PubMed] [Google Scholar]
- 63.Zeng, Z., D. D. Sarbassov, I. J. Samudio, K. W. Yee, M. F. Munsell, C. E. Jackson, F. J. Giles, D. M. Sabatini, M. Andreeff, and M. Konopleva. 2007. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109:3509-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, Z., W. H. Leister, R. G. Robinson, S. F. Barnett, D. Defeo-Jones, R. E. Jones, G. D. Hartman, J. R. Huff, H. E. Huber, M. E. Duggan, and C. W. Lindsley. 2005. Discovery of 2,3,5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorg. Med. Chem. Lett. 15:905-909. [DOI] [PubMed] [Google Scholar]