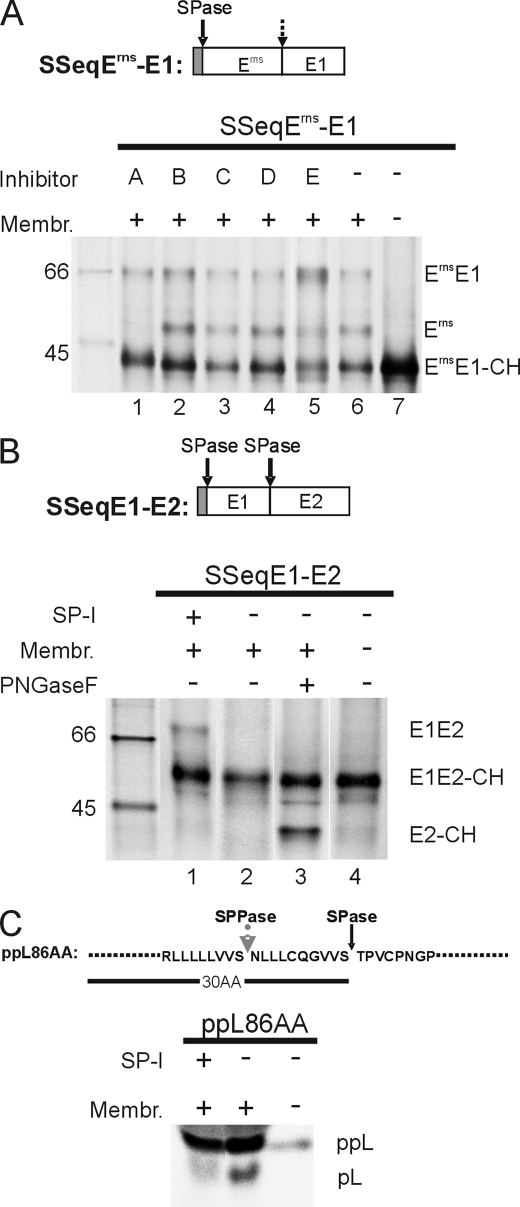
FIGURE 4.
Influence of different protease inhibitors on ErnsE1 processing. A, SDS-PAGE with products of in vitro translation experiments with construct SSeqErns-E1 (shown on top) in the presence of the protease inhibitors specified by letters above the gel (A, SP-I, MeOSuc-Ala-Ala-Pro-Val chloromethyl ketone used at 1.5–2.5 mm; B, signal peptide peptidase inhibitor, 1,3-di-(N-carboxybenzoyl-l-leucyl-l-leucyl)amino acetone, used at 10 μm; C, γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, used at 100 μm; D, serine/cysteine protease inhibitor, leupeptin, used at 25 μm; E, site-2 protease-metalloprotease inhibitor, 1,10-phenanthroline, used at 5 mm). Note that the concentration at which 1,10-phenanthroline was used in this study was shown to inhibit also the site 1 intramembrane protease (71). Translation of the RNA in the absence of xinhibitor or in the absence of inhibitor and microsomal membranes, as specified by the code at the top of the gel, served as controls. Lane 5, showing the products obtained in the presence of inhibitor E, was exposed 5 times longer than the other lanes. See also the legend to Fig. 1. Effects of SP-I on processing of construct SSeqE1-E2 (B) or a preprolactin construct (C) were also determined. For the preprolactin construct, which includes the first 86 amino acids of the precursor protein, the signal peptide of 30 amino acids with the SPase and the signal peptide peptidase (SPPase) cleavage sites are specified. Protein size marker bands are shown with the molecular masses given in kDa.