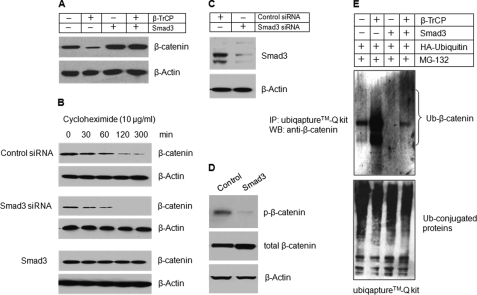
FIGURE 2.
Smad3 protects β-catenin degradation in chondrocytes. A, Smad3 expression plasmid was co-transfected with β-TrCP into RCJ3.1C5.18 cells. 24 h after transfection, the cell lysates were collected, and Western blotting (WB) was performed using the anti-β-catenin antibody. Transfection of Smad3 prevented β-catenin degradation. B, RCJ3.1C5.18 cells were transfected with control or Smad3 siRNA or Smad3 expression plasmid. The cell lysates were collected 0, 30, 60, 120, or 300 min after cycloheximide treatment (10 μg/ml), and the β-catenin protein levels were detected by Western blotting. Silencing of Smad3 accelerated β-catenin degradation and transfection of Smad3 prevented β-catenin degradation. C, the silencing efficiency of Smad3 siRNA was determined by Western blotting. The efficiency is over 70%. D, RCJ3.1C5.18 cells were transiently transfected with Smad3 expression plasmid. 24 h after transfection, the cell lysates were collected, and Western blotting was performed using the anti-p-β-catenin antibody. Smad3 significantly inhibited β-catenin phosphorylation. E, Smad3 and HA-ubiquitin expression plasmids were co-transfected with β-TrCP expression plasmid into RCJ3.1C5.18 cells in the presence of proteasome inhibitor MG132 (10 μm, 4 h of incubation). 24 h after transfection, the cell lysates were collected, and ubiquitinated proteins were pulled down using an UbiQaptureTM-Q kit, and ubiquitinated β-catenin was detected using the anti-β-catenin antibody. Transfection of β-TrCP induced β-catenin ubiquitination (second lane) and co-transfection of Smad3 prevented β-catenin ubiquitination (fourth lane). Ub, ubiquitin; IP, immunoprecipitation.