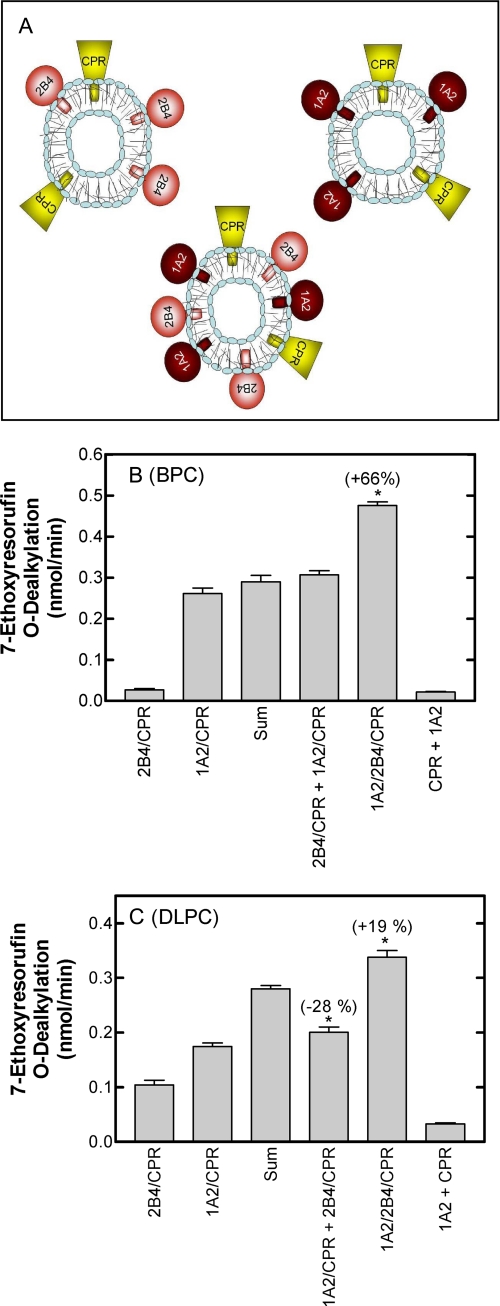
FIGURE 3.
Interactions among CYP1A2, CYP2B4, and CPR require the proteins to reside in the same vesicles. A, this schematic describes the experimental strategy used to demonstrate physical interactions between CYP1A2 and CYP2B4. The figure shows three different types of reconstituted systems represented by the P450s (either CYP2B4 or CYP1A2) and CPR. Substrate metabolism was compared using a mixture of the two binary systems containing CPR and one of the two P450 enzymes (the two upper systems in the panel) and a ternary system containing the CPR and both P450 enzymes (lower system in the panel). B, the rates of 7ER metabolism by binary, mixed binary, and ternary reconstituted systems of CYP1A2, CYP2B4, and CPR in BPC. The rate of 7ER metabolism by binary systems of CYP2B4 and CPR (2B4/CPR) and CYP1A2 and CPR (1A2/CPR), mixed binary systems (2B4/CPR + 1A2/CPR), and the ternary system (1A2/2B4/CPR) was measured in BPC using the glycocholate-detergent method for preparation of the reconstituted system. In addition, the sum of the means of the rates by the binary systems is shown to represent the rate that would be expected by the mixed systems if there was no interaction between the P450 enzymes that influenced metabolism. A negative control of a mixture of the simple systems in which the CPR and the P450 enzyme were incorporated in separate lipid vesicles is also shown (1A2 + CPR). *, a significant difference from the “sum” group (p < 0.05). C, rates of 7ER metabolism by binary, mixed binary, and ternary reconstituted systems of CYP1A2, CYP2B4, and CPR in DLPC. Reconstituted systems were the same as described in B except that the proteins were reconstituted into preformed DLPC vesicles. A negative control of a mixture of the simple systems in which the CPR and the P450 enzyme were incorporated in separate lipid vesicles is also shown (1A2 + CPR).