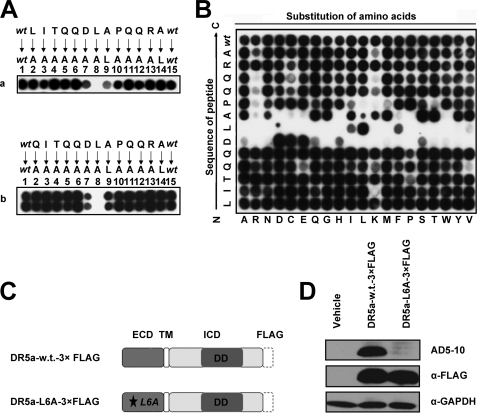
FIGURE 2.
Residues 5DLA7 are essential for the interaction between DR5 and AD5-10. A, alanine-scanning mutagenesis of the epitope. Two alanine-scanning arrays of peptides −1LITQQDLAPQQRA12 (panel a) and QI1TQQDLAPQQRA12 (panel b) were probed with 0.2 μg/ml AD5-10. The sequences of the peptides are shown. The first and the last spot corresponded to the parental peptide, whereas the other spots represented an Ala-substituted analogue of wild-type sequence (wt) as indicated above the individual spots. B, permutation array of peptide −1LITQQDLAPQQRA12. Each residue in this peptide was replaced, one at a time, by a naturally occurring amino acid. The resulting array of 260 (13 × 20) peptides was probed with 0.2 μg/ml of AD5-10. Spots that displayed the “half-moon” pattern were likely to be caused by imperfections in array synthesis. C, FLAG-tagged full-length DR5. A C-terminal FLAG tag was added to the full-length DR5. D, analysis of point mutation in full-length DR5 molecule. Both wild-type and mutant FLAG-tagged DR5 constructs were introduced into HEK293T/17 cells by transient transfection. Cell lysates were probed by immunoblot analysis using AD5-10 or anti-FLAG mAb. All experiments were repeated at least three times with similar results. TM, transmembrane; w.t., wild type; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.