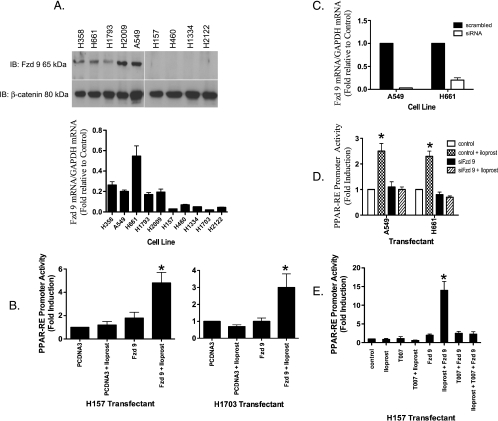
Figure 2.
RNAi knockdown of Fzd 9 results in reduced PPARγ activity expression. (A) Total RNA purified from the indicated NSCLC cell lines were submitted to quantitative RT-PCR using primers specific for Fzd 9 as described under Materials and Methods. The relative mRNA abundance for Fzd 9 in the different samples was normalized to human GAPDH measured by RT-PCR in the same samples. Aliquots of extracts containing equal protein as measured by the Bradford assay from the indicated cells were resolved by SDS-PAGE and immunoblotted with antibodies to Fzd 9 (100 kDa; Aviva Systems Biology). The filters were stripped and reimmunoblotted for β-catenin (80 kDa; BD Transduction Laboratories) as a loading control. (B) The cell lines H157 and H1703 were transiently transfected with or without Fzd 9 and PPAR-RE along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (C) siRNA knockdown of Fzd 9 was performed as described in Materials and Methods on the indicated cell lines. Total RNA purified from these cells were then submitted to quantitative RT-PCR using primers specific for Fzd 9 as previously described. The relative mRNA abundance for Fzd 9 in the different samples was normalized to human GAPDH. (D) The indicated cell lines were transiently transfected with PPAR-RE, along with CMV-β-gal to normalize for transfection efficiency. In addition, Fzd 9 was knocked down by transient siRNA expression as indicated previously. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (E) The indicated cell line was transiently transfected with PPAR-RE, empty vector, or Fzd 9 along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost and/or 5 µM T0070907.