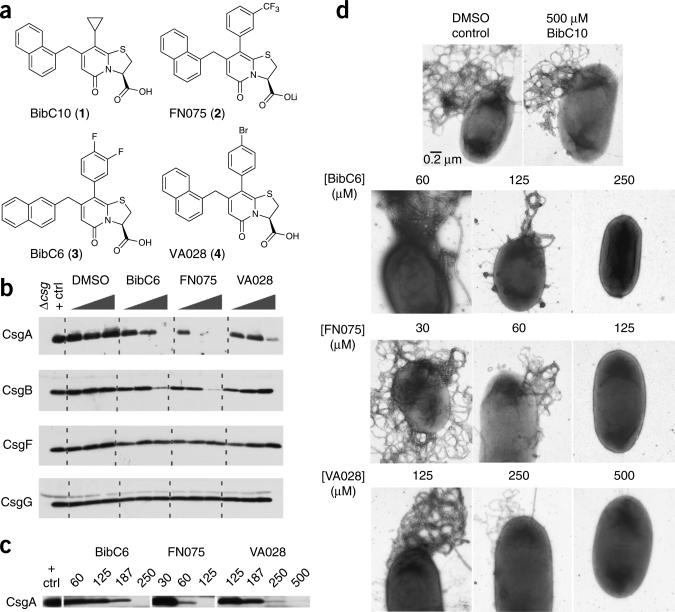
Figure 1.
Curlicide inhibition of in vivo amyloid biogenesis in E. coli. (a) BibC10 (1), FN075 (2), BibC6 (3) and VA028 (4) are ring-fused 2-pyridones that differ in their phenyl ring modifications and, for 3, in the position of naphthyl substitution. (b) CsgA, CsgB, CsgF and CsgG protein profiles obtained by western analysis of MC4100 grown on curlicide-amended agar for 48 h. Each curlicide was tested at 0.1, 1.0 and 2.5 mM, and the positive control sample corresponds to cells grown in DMSO-amended agar. MC4100Δcsg was the negative control. Curli production was abolished at 1.0 mM compound 2, 2.5 mM compound 3. Only a faint CsgA band was observed at 2.5 mM compound 4. (c) Curlicides were effective at the designated micromolar concentrations in blocking curli biogenesis in UTI89 growing in shaking YESCA broth (100 rpm) for 48 h at 28 °C. (d) Representative high-resolution EM images of UTI89 prepared as in c. Titratable reductions in bacterial curliation were observed for cells grown in the presence of curlicides. The scale bar in the first electron micrograph represents 0.2 μm and applies to all images.