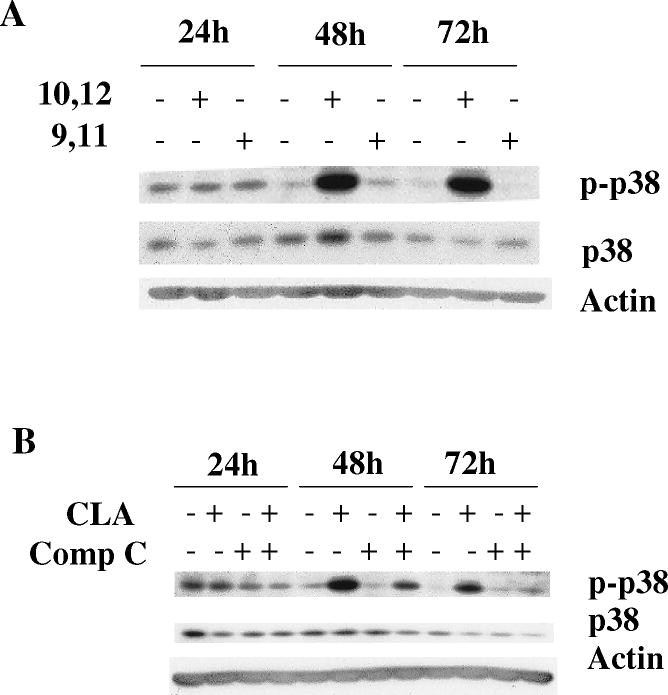
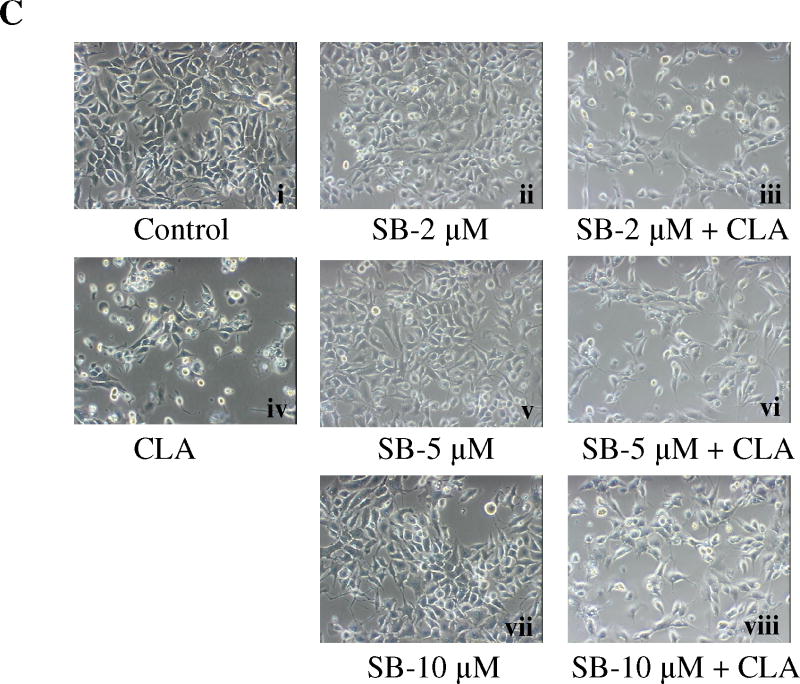
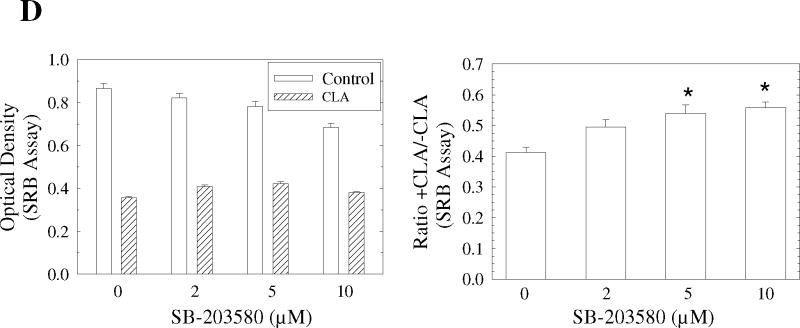
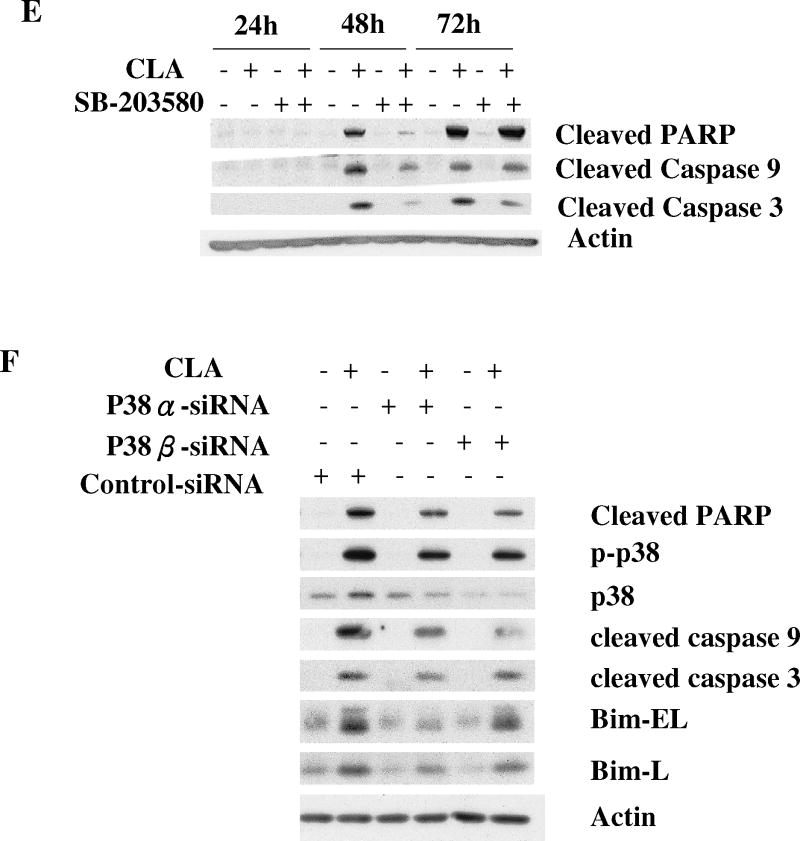
Figure 5.
Activation of p38 MAPK signaling plays an important role in CLA-induced apoptosis. TMT4t cells were treated with or without 40 μM CLA in the absence or presence of the indicated inhibitors for 24, 48 or 72 hr, and the samples prepared for western blot analysis. A. t10,c12-CLA, but not c9,t11-CLA stimulates phosphorylation of p38. B. The AMPK inhibitor compound C (20 μM) attenuates the t10,c12-CLA-induced phosphorylation of p38. C and D. TM4t cells were treated for 72 hr with or without 40 μM of t10,c12-CLA and/or various concentrations of SB-203580 (SB), photographed under the 10× microscope objective (C), then viable cell number estimated by the SRB assay on the attached (viable) cell monolayer (D). The data in the right panel of D show the ratio of the ODs from the SRB assay from CLA-treated to control cells. The asterisks denote a statistically significant difference from the control group. E. The p38 inhibitor SB-203580 (10 μM) attenuates t10,c12-CLA-induced cleavage of PARP, caspase 3 and caspase 9. F. p38 siRNAs attenuate t10,c12-CLA-induced cleavage of PARP, caspase 3 and caspase 9, as well as the t10,c12-CLA induction of Bim. TM4t cells were transfected with p38α, p38β or control siRNA for 6 hr followed by treatment with t10,c12-CLA for 72 hr.