Fig. 3.
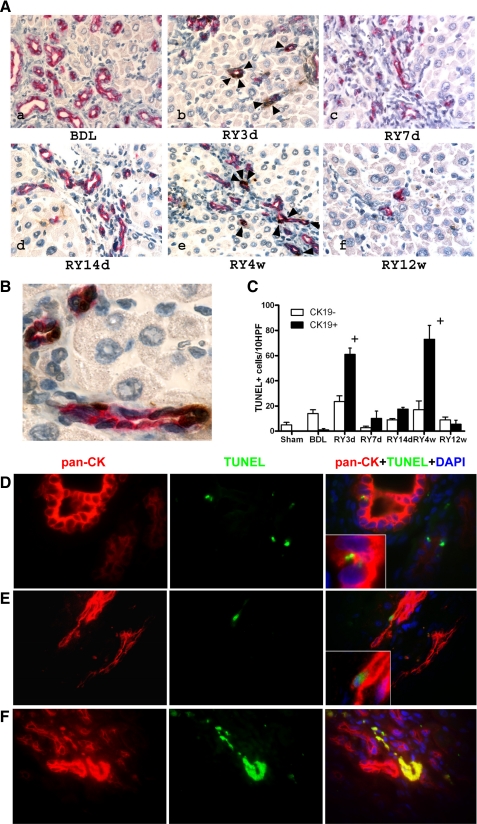
Cholangiocyte apoptosis is a prominent feature of biliary fibrosis reversal. A: double staining for bile duct epithelial (cytokeratin 19, CK19) and apoptotic cells (TdT-mediated dUTP nick end labeling, TUNEL) demonstrates a sharp increase in double-positive cells 3 days (b) and 4 wk (e) after RY-anastomosis (arrows), whereas there are no apoptotic cholangiocytes at peak fibrosis (a). Original magnification: ×200. B: high-power magnification (×600) of a representative section 4 wk after RY-anastomosis when maximal apoptosis occurs, demonstrating that almost all CK19+ cholangiocytes (red staining) forming bile ducts are TUNEL positive (brown nuclear staining), whereas other portal cells and hepatocytes are TUNEL negative (light blue nuclear counterstain). C: quantification of apoptosis: CK19-negative/TUNEL-positive (open bars) and CK19/TUNEL-double-positive cells (shaded bars) were counted in 3–5 animals per group in at least 10 random fields at a magnification of ×200. Numbers are expressed as positive cells/10 high-power fields (HPF), means ± SE. D–F: detection of apoptotic cholangiocytes by immunofluorescent double-labeling for TUNEL and the alternative cholangiocyte marker pan-cytokeratin (pan-CK), confirming the results obtained using TUNEL/CK-19 double immunohistochemistry. D: no detectable cholangiocyte apoptosis is found at the peak of fibrosis (BDL4w). Small TUNEL-positive nuclei are rare and belong to intraepithelial (inflammatory) cells in proliferating bile ducts (inset). E: early apoptotic cholangiocyte at the peak of resolution (RY4w) identified by the large TUNEL+ nucleus, pan-CK+ cytoplasm, typical morphology and its location within a bile duct (inset). F: late apoptotic cholangiocytes within a vanishing bile duct. TUNEL+, p-CK+ material demonstrates colocalization in the merged image (yellow). Original magnification, ×100.