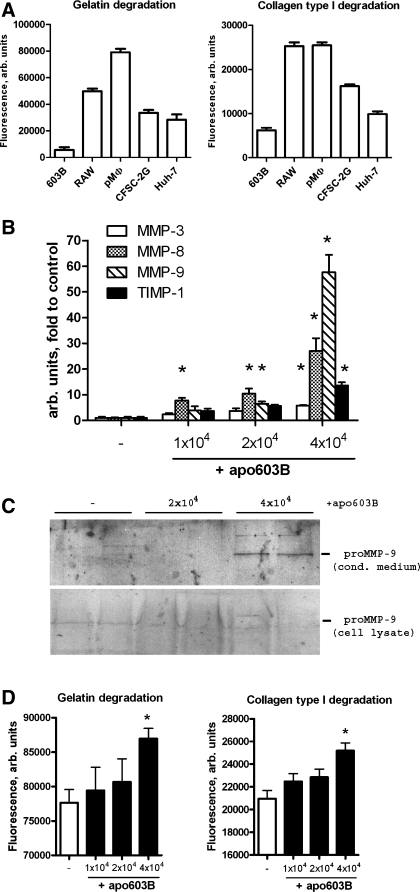
Fig. 6.
Engulfment of apoptotic cholangiocytes induces MMP expression and matrix-degrading activities in macrophages. A: high intrinsic gelatinase and collagenase activity in macrophage vs. nonmacrophage cell cultures. Freshly isolated rat peritoneal macrophages (pMΦ), the murine cholangiocyte cell line (603B), the rat hepatic stellate cell line CFSC-2G, and human hepatoma cells (Huh-7) were cultured at a density of 30 × 104 cells/well in the presence of self-quenched DQ-gelatin or DQ-collagen substrates for 16 h to measure live cell gelatinase and collagenase activities as described in materials and methods. B: dose-dependent induction of MMP expression in macrophages upon engulfment of apoptotic cholangiocytes. Apoptotic 603B cholangiocytes (apo603B) were prepared as described in materials and methods, and 1–4 × 104 cells were added to freshly isolated rat peritoneal macrophages (3 × 104 cells/well). Relative mRNA levels of MMP-3, -8, and -9 and TIMP-1 were determined by quantitative RT-PCR 12 h after addition of apoptotic cells. C: corresponding increase in secreted MMP-9 protein levels upon phagocytosis as detected by Western blotting of conditioned macrophage supernatants; no MMP-9 detected in the corresponding cell lysate. D: phagocytosis-associated MMP induction results in a net increase in macrophage matrix-degrading activity. Collagenolytic and gelatinolytic activities were determined as outlined above over 16 h after addition of apoptotic 603B cholangiocytes at increasing concentrations. Data are expressed as means ± SE, with each bar representing 3 (B) or 6–8 (A and D) wells per experimental condition. All in vitro experiments were repeated at least 3 times. *P < 0.05 compared with the control group.