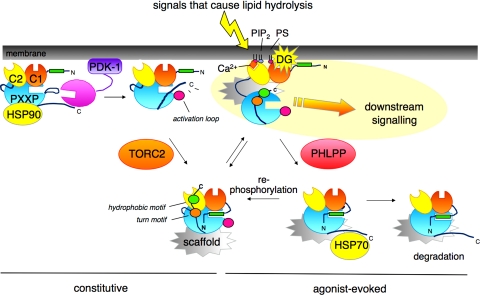
Fig. 3.
Model for the life cycle of PKC, from biosynthesis to degradation. Newly synthesized PKC (leftmost species) associates with a membrane fraction, where it is processed by a series of ordered and tightly coupled phosphorylations. Heat shock protein-90 (HSP90) binds to the PXXP clamp in the kinase domain right before the CT (see text), an event required for priming phosphorylations. Two upstream kinases control priming phosphorylations: PDK-1, bound to the exposed carboxyl terminus of newly synthesized PKC, phosphorylates the activation loop (pink circle); this step appears to be first, and necessary for the processing PKC. The mTORC2 complex promotes the phosphorylation of the turn motif (orange circle), second phosphorylation event, and hydrophobic motif (green circle), the final phosphorylation event. The fully phosphorylated “mature” PKC localizes to the cytosol with the pseudosubstrate (green rectangle) occupying the substrate-binding cavity (bottom species on left). Signals that cause lipid hydrolysis recruit PKC to membranes. For conventional PKC isozymes, binding of Ca2+ to the C2 domain recruits them to the plasma membrane via interaction with PIP2, an event that allows efficient binding of the membrane-embedded ligand DG (top right species). For novel PKC isozymes, the intrinsic affinity of the C1 domain is sufficiently high to allow direct recruitment to membranes by agonist-evoked levels of DG. Membrane-bound PKC adopts an open conformation, in which the pseudosubstrate is released from the kinase domain, allowing downstream signaling (top right species). This open conformation is sensitive to dephosphorylation: the phosphatase PHLPP (PH domain leucine-rich repeat protein phosphatase) dephosphorylates the hydrophobic motif, an event that shunts PKC to the detergent-insoluble fraction where it is further dephosphorylated and degraded (bottom right species). Note: molecular chaperone HSP70 binds the dephosphorylated turn motif (bottom middle species), an event that promotes rephosphorylation of PKC and reentry into the pool of signaling-competent enzyme, thus sustaining the lifetime of the enzyme. Specific protein scaffolds (gray structures shown associated with matured PKC) bind specific isozymes and specific species [e.g., RACKs bind active PKC, but other scaffolds bind inactive PKC] to poise PKC in specific cellular microdomains. The amplitude of the PKC signal and similarly the responsiveness of the cell to low concentrations of agonist are ultimately controlled by how much PKC is in the cell.