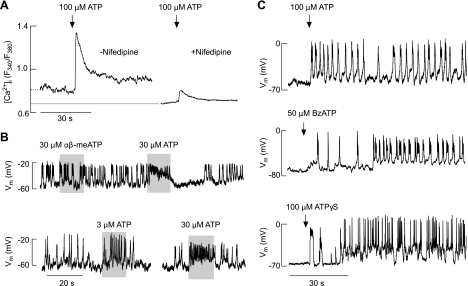
Fig. 3.
Agonist-induced changes in Ca2+ signaling and electrical activity in TRH-responsive cells. A: ATP induced an elevation in intracellular Ca2+ concentration ([Ca2+]i) in cells bathed in medium containing MRS2500, a specific inhibitor of P2Y1Rs. Experiments were done in Ca2+-containing medium in the absence (left trace) and presence (right trace) of nifedipine, a specific blocker of L-type voltage-gated Ca2+ channels during the initial agonist application. Nifedipine (1 μM) was kept for 10 min before ATP application. Traces shown are mean values for several cells in 2 dishes (with and without nifedipine) in 1 of several experiments. Dotted line denotes basal [Ca2+]i in the presence and absence of nifedipine. The averaged basal [Ca2+]i (expressed as the fluorescence intensity ratio F340/F380) was 0.77 ± 0.05 (n = 13) and 0.63 ± 0.04 (n = 12) in the absence and presence of nifedipine, respectively (P < 0.05). ATP-induced peak [Ca2+]i response was 1.29 ± 0.12 (n = 13) and 0.79 ± 0.05 (n = 12) in the absence and presence of nifedipine, respectively (P < 0.05). B: modulation of patterns of electrical activity in spontaneously firing cells. Note the lack of effects of αβ-methylene ATP (αβ-meATP), a specific agonist for P2X1R and P2X3R, in an ATP-responsive cell (top) and dose-dependent effects of ATP on the frequency of firing (bottom). Vm, membrane voltage potential. C: initiation of firing of action potentials in quiescent cells by ATP (top), 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-trisphosphate (BzATP; middle), and adenosine 5′-O-(3-thiophosphate) (ATPγS; bottom).