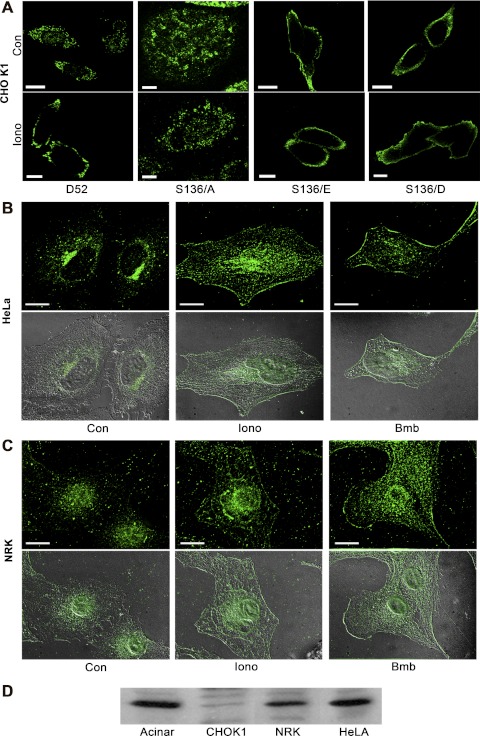
Fig. 2.
Phosphorylation at serine 136 mediates D52 accumulation on the plasma membrane. A: a single confocal optical section of CHO-K1 cells transfected with Wt-D52 or the indicated S136 mutants that were treated as control or with 2 μM ionomycin for 5 min prior to fixation in 2% formaldehyde. D52 immunoreactivity was detected in A by using anti-hemagglutinin (HA) tag (1:100) with Alexa Fluor 546-conjugated anti-mouse IgG (1:500). Note the pronounced accumulation of Wt-D52 to the plasma membrane in response to elevated cellular Ca2+, which was abolished in the S136/A mutants. Likewise, note the pronounced accumulation of phosphomimetic mutants along the plasma membrane independent of elevated Ca2+. B and C: endogenous D52 (1:100) was analyzed in HeLa and normal rat kidney (NRK) cells treated as control or stimulated with 2 μM ionomycin or 100 nM of the bombesin analog Lys3-bombesin (Bmb) for 5 min and detected by use of Alexa Fluor 488-conjugated anti-rabbit IgG (1:500). Images are a reconstructed z-series acquired by brightfield microscopy. Corresponding differential interference microscopy (DIC) images are shown below each immunofluorescence image. All images are representative of multiple determinations performed on at least 3 separate tissue preparations. Bars, 20 μm. D: immunoblot of D52 (1:1,000) in lysates (100 μg) from pancreatic acinar cells, CHO-K1, NRK, and HeLa cells. Note that acinar, NRK, and HeLa cells express significant levels of endogenous D52, whereas CHO-K1 cells exhibit much less immunoreactivity.