Abstract
The content of meals consumed after exercise can impact metabolic responses for hours and even days after the exercise session. The purpose of this study was to compare the effect of low dietary carbohydrate (CHO) vs. low energy intake in meals after exercise on insulin sensitivity and lipid metabolism the next day. Nine healthy men participated in four randomized trials. During the control trial (CON) subjects remained sedentary. During the other three trials, subjects exercised [65% peak oxygen consumption (V̇o2peak); cycle ergometer and treadmill exercise] until they expended ∼800 kcal. Dietary intake during CON and one exercise trial (BAL) was designed to provide sufficient energy and carbohydrate to maintain nutrient balance. In contrast, the diets after the other two exercise trials were low in either CHO (LOW-CHO) or energy (LOW-EN). The morning after exercise we obtained a muscle biopsy, assessed insulin sensitivity (Si; intravenous glucose tolerance test) and measured lipid kinetics (isotope tracers). Although subjects were in energy balance during both LOW-CHO and CON, the lower muscle glycogen concentration during LOW-CHO vs. CON (402 ± 29 vs. 540 ± 33 mmol/kg dry wt, P < 0.01) coincided with a significant increase in Si [5.2 ± 0.7 vs. 3.8 ± 0.7 (mU/l)−1·min−1; P < 0.05]. Conversely, despite ingesting several hundred fewer kilocalories after exercise during LOW-EN compared with BAL, this energy deficit did not affect Si the next day [4.9 ± 0.9, and 5.0 ± 0.8 (mU/l)−1·min−1]. Maintaining an energy deficit after exercise had the most potent effect on lipid metabolism, as measured by a higher plasma triacylglycerol concentration, and increased plasma fatty acid mobilization and oxidation compared with when in nutrient balance. Carbohydrate deficit after exercise, but not energy deficit, contributed to the insulin-sensitizing effects of acute aerobic exercise, whereas maintaining an energy deficit after exercise augmented lipid mobilization.
Keywords: carbohydrate, glycogen, insulin resistance, triglyceride, fatty acid
many of the improvements in metabolic health associated with exercise stem largely from the most recent session of exercise, rather than from an increase in “fitness,” per se (6, 15, 20). Importantly, the content of meals ingested after exercise can have a tremendous impact on the magnitude and duration of the metabolic responses that occur in the hours and even days after exercise (4, 5). Therefore, when trying to interpret the metabolic responses to exercise, and perhaps more importantly the mechanisms underlying these responses, it is critical to control (or at least take into consideration) the components and content of the meals ingested afterward. Careful control of macronutrient intake, carbohydrate in particular, is very important when studying exercise-induced metabolic responses. However, the metabolic alterations specifically associated with an “energy deficit” (i.e., consuming less energy than expended) vs. a “carbohydrate deficit” (i.e., consuming less carbohydrate than expended) are not well understood.
It is well established that a single session of exercise can enhance insulin action, even for the next 1–2 days after exercise (4, 5, 22, 25). Much of this exercise-induced improvement in insulin sensitivity has been linked with the reduction in muscle glycogen that occurs during exercise (4, 5, 22, 25, 27, 35). Accordingly, a high carbohydrate intake after exercise accelerates the restoration of muscle glycogen stores and rapidly reverses the exercise-induced improvement in insulin sensitivity (4, 5, 19). Conversely, a low intake of carbohydrate after exercise that maintains a low muscle glycogen concentration after exercise can prolong the insulin-sensitizing effects (4, 5). Alternatively, the effect of an exercise-mediated energy deficit on insulin sensitivity is less clear. Much of this uncertainty is a consequence of the fact that it is not possible to create an energy deficit after exercise without also creating a “deficit” in at least one of the three major macronutrients, making it impossible to specifically tease out the independent effects of an energy deficit. Importantly, although many metabolic processes are acutely sensitive to even small changes in dietary carbohydrate, evidence suggests the same is not true for changes in fat availability. Therefore, inducing an energy deficit after exercise by removing only dietary fat may be a more effective model for isolating the effects of an energy deficit on insulin sensitivity. Improving our understanding about how different dietary treatments after exercise affect insulin sensitivity will guide recommendations to help maximize the insulin-sensitizing effects after each exercise session.
A single session of exercise can also alter lipid metabolism. Indeed, a prior session of exercise has been reported to increase resting fat oxidation (17, 29, 34), improve blood lipid profile (1, 32), and lower the plasma triglyceride response after a meal (1, 32, 33). While these findings are not universal, some of this disparity can likely be explained by differences in nutrients ingested in the hours after exercising (14, 17). Still, the impact of changes in dietary carbohydrate, and/or energy content in meals after exercise on exercise-induced alterations in lipid metabolism is not well known. Therefore, the primary aims of this study were to compare the effects of an exercise-induced energy deficit vs. carbohydrate deficit on insulin sensitivity and lipid metabolism the next day. We hypothesized that when compared with no exercise, being in a carbohydrate deficit after exercise (without an energy deficit) would increase insulin sensitivity and suppress plasma triglyceride concentration the next morning. Additionally, we hypothesize that being in an energy deficit after exercise (without a carbohydrate deficit) would not affect insulin sensitivity but would increase fatty acid mobilization and oxidation the next morning.
SUBJECTS AND METHODS
Subjects
Nine nonobese [body mass index (BMI) ≤ 25 kg/m2], sedentary men (<2 h activity/wk for ≥6 mo) participated in this study [age 29 ± 1 yr old, body mass 75.2 ± 1.1 kg, BMI 23.6 ± 0.3 kg/m2, body fat 20.1 ± 0.8%, peak oxygen consumption (V̇o2peak) 36.9 ± 1.0 ml·kg−1·min−1]. Subjects were not taking any medications, and all were considered to be in good health after a comprehensive medical examination that included medical history, a physical examination, blood tests, and an electrocardiogram. Any history of metabolic or cardiovascular disease resulted in exclusion from participation. Subjects were nonsmokers and had been weight stable (±2 kg) for ≥6 mo before participation. Written informed consent was obtained from all subjects before initiating participation. All procedures of this study were approved by the University of Michigan Institutional Review Board.
Preliminary Testing
Before beginning the study we measured body composition using dual-energy X-ray absorptiometry (Lunar Prodigy Advance; GE Healthcare, Little Chalfont, Buckinghamshire, UK) for determination of whole body fat mass (FM) and fat-free mass (FFM). V̇o2peak was also measured before the study using a cycle ergometer exercise protocol (Examiner; Lode BV, Groningen, the Netherlands; Max II; Physio-Dyne Instrument, Quogue, NY). This protocol consisted of a 3-min warm-up, followed by a progressively increasing work rate until volitional fatigue (∼10 min). This preliminary exercise test was performed at least 1 wk before the subjects' first experimental trial.
Overall Study Design
Subjects were admitted to the Michigan Clinical Research Unit in the University of Michigan Hospital for a 2-day experiment on four separate, randomized occasions separated by ≥7 days. During three of these visits, subjects exercised at moderate intensity (60–65% V̇o2peak) for ∼90 min on day 1 of their 2-day stay. These exercise trials differed only in the content of the meals ingested after exercise. During one exercise trial, meals after exercise provided adequate nutrients to replace the carbohydrate and total energy expended during exercise, and therefore subjects were considered to be in nutrient balance (BAL). In contrast, during the other two exercise trials the meals after exercise were deficient in either carbohydrate (LOW-CHO) or total energy (LOW-EN). During the control trial (CON), subjects did not exercise and were fed meals to maintain nutrient balance (see details in Study Diets). On all occasions subjects stayed overnight in the hospital, and the next morning we performed a battery of metabolic tests (see details below).
Experimental Protocol
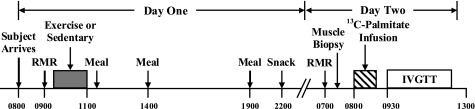
A timeline describing the events of the experimental trials is presented in Fig. 1. Subjects were admitted to the hospital in the morning after an overnight fast, and then they rested quietly in bed for 30 min. At 0900 oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured (indirect calorimetry, ventilated hood; Vmax Encore; CareFusion, San Diego, CA) for calculation of resting energy expenditure. During three of the trials subjects began exercise at 0930 at moderate intensity (60–65% V̇o2peak). For all three exercise sessions, we required subjects to expend 14 kcal/kg FFM (∼800 kcal; ∼90 min of exercise), whereas subjects remained sedentary during the control trial (CON). The exercise consisted of ∼45 min of treadmill exercise followed immediately by ∼45 min of cycle ergometer exercise. To ensure subjects were exercising at the appropriate intensity and to quantify energy expenditure during exercise, V̇o2 and V̇co2 were measured during minutes 0–10 and 30–35 of each mode of exercise, and appropriate adjustments to exercise intensity were made if necessary. It has been found that exercising at a similar intensity and duration reduced muscle glycogen concentration ∼55% (7). Meals were ingested after exercise (or after resting quietly for 90 min during CON) at 1130, 1400, and 1900 (see Study Diets). To control for any effect of the last meal on measurements made the subsequent morning, subjects consumed the same snack at the end of the evening (2200) during all trials.
Fig. 1.
Timeline of trial events. Subjects performed 4 overnight trials in a randomized order (1 sedentary trial and 3 exercise trials). The exercise trials were identical with the exception of dietary intake (see Study Diets). RMR, resting metabolic rate; IVGTT, intravenous glucose tolerance test.
The next morning we measured resting V̇o2 and V̇co2 at 0630 to calculate rates of fatty acid oxidation the day after each treatment. At this time, expired breath samples were also collected from a mixing chamber to determine background isotopic enrichment of 13CO2. At 0700 one intravenous catheter was placed into the antecubital vein for use as an infusion line and another retrograde intravenous catheter was placed into a contralateral hand vein for sampling of arterialized blood using the heated-hand technique (18). A skeletal muscle biopsy was obtained from the vastus lateralis at 0730. Muscle samples were quickly dissected free of adipose and connective tissue, rinsed in saline, blotted dry, and then frozen in liquid nitrogen. Beginning at 0800, a constant infusion (0.04 μmol·kg−1·min−1) of [1-13C]palmitate (Cambridge Isotopes, Andover, MA) bound to human albumin with a [13C]bicarbonate prime (1.2 μmol/kg; Cambridge Isotopes) was started and continued for 60 min to determine palmitate uptake and appearance. Both before (at 0750, 0755, 0800) and during the infusion (at 0850, 0855, 0900) blood was sampled from the heated hand vein for determination of background and steady-state plasma [13C]palmitate enrichment, respectively. At 0845 resting V̇o2 and V̇co2 were reassessed, and expired breath samples were again collected for steady-state isotopic enrichment of 13CO2 for calculation of plasma [13C]palmitate oxidation. Beginning at 0930, an intravenous glucose tolerance test (IVGTT) was conducted to assess insulin sensitivity using the minimal model method (2). Briefly, subjects were injected with a bolus of glucose (300 mg/kg) at 0930, and 20 min later with a bolus of insulin (0.02 IU/kg). Blood was sampled immediately before and frequently for 3 h after the glucose injection.
Study Diets
The energy content and macronutrient breakdown of the different diets are provided in Table 1. To maintain energy balance during CON (i.e., no exercise performed), subjects were provided a total of 38 kcal/kg FFM (26) from the three meals (ingested at 1130, 1400, and 1900) and one snack (at 2200). Because subjects expended 14 kcal/kg FFM during the exercise sessions, to achieve energy balance during BAL and LOW-CHO subjects were provided a total of 52 kcal/kg FFM in the meals ingested after exercise. Alternatively, to maintain an energy deficit after exercise during LOW-EN, subjects were only fed a total of 38 kcal/kg FFM. Accordingly, during LOW-EN fat ingestion was kept very low (0.28 g fat/kg FFM; ∼15 g fat in total), while the other macronutrients were matched with BAL. To ensure adequate glycogen restoration after exercise during BAL and LOW-EN, carbohydrate intake was increased to a total of ∼450 g during these trials (7.7 g CHO/kg FFM). Conversely, to achieve a carbohydrate deficit after exercise during LOW-CHO, carbohydrate ingestion was kept relatively low (3.5 g CHO/kg FFM; ∼200 g CHO in total) while fat ingestion was increased (3.7 g fat/kg FFM; ∼200 g fat in total) to maintain energy balance. Protein intake (1.15 g protein/kg FFM; ∼70 g protein in total) was identical during all trials. The nighttime snack that subjects ate at 2200 was identical in each trial (i.e., ∼175 kcal; 60% CHO, 25% fat, 15% protein) to prevent the confounding influence of different contents of the last meal on metabolic responses the next day (28). The energy content and macronutrient breakdown for each diet were evenly distributed among the meals provided on day 1 of the study.
Table 1.
Experimental diets
| CON | BAL | LOW-EN | LOW-CHO | |
|---|---|---|---|---|
| Carbohydrate, g | 299 ± 10 | 461 ± 16 | 460 ± 18 | 210 ± 8 |
| Fat, g | 86 ± 3 | 112 ± 4 | 17 ± 1 | 221 ± 8 |
| Protein, g | 69 ± 2 | 70 ± 2 | 70 ± 3 | 70 ± 2 |
| Energy intake, kcal | 2,250 ± 78 | 3,138 ± 107 | 2,268 ± 90 | 3,091 ± 110 |
Values are means ± SE. CON, control trial; BAL, subjects in nutrient balance; LOW-EN, low energy intake; LOW-CHO, low carbohydrate intake.
Analytical Procedures
Blood sampling and plasma metabolite and insulin concentrations.
All blood samples were collected in tubes containing 0.03 mmol EDTA. Samples were then centrifuged at 1,600 g for 20 min; plasma was separated and then stored at −80°C until later analysis. Plasma glucose (Glucose Oxidase; Thermo Fisher Scientific, Waltham, MA), fatty acid (HR Series NEFA; Wako Chemicals USA, Richmond, VA), and triacylglycerol (Triglyceride Reagent; Sigma Aldrich, St. Louis, MO) concentrations were measured by colorimetric assay. Plasma insulin concentration was measured by radioimmunoassay (Human Insulin-specific RIA; Millipore, Billerica, MA).
Muscle glycogen and triacylglycerol concentration.
Muscle biopsy samples were lyophilized at −60°C for 48 h, and aliquots were weighed to the nearest 0.1 mg. Muscle glycogen was determined from the measurement of glucose after acid hydrolysis as previously described (24). Briefly, samples were homogenized and then hydrolyzed in 2 N HCl and heated at 100°C for 2 h. Samples were then neutralized using 1 N NaOH to pH 6.5–7.5, and the free glucose concentration was determined fluorometrically. Intramyocellular triacylglycerol (IMTG) was measured from the liberation of free glycerol as previously described (10). Briefly, triacylglycerols were extracted from the dried muscle sample using a 2:1 chloroform:methanol solution and saponified in 4% ethanolic KOH. Free glycerol concentration was then determined fluorometrically.
Tracer enrichment in plasma palmitate and breath CO2.
The tracer-to-tracee ratio (TTR) for plasma palmitate was determined by gas chromatography-mass spectrometry (GC/MS; Agilent 5973Networks, Mass Selective Detector; Agilent Technologies, Palo Alto, CA). Briefly, proteins were precipitated from plasma samples with acetone, and hexane was used to extract plasma lipids. Fatty acids were converted to their methyl esters with iodomethane and isolated by using solid-phase extraction cartridges (Sigma Aldrich). After electron impact ionization, ions with mass-to-charge ratios 270.2 and 271.2 were selectively monitored on the GC/MS. The ratio of 13CO2 to 12CO2 in expired breath was determined by isotope ratio mass spectrometry (Finnigan MAT, Bremen, Germany). Briefly, water was removed from the sample via passage through a desiccant column. CO2 was then isolated, and the mass ratio of 13CO2 to 12CO2 (mass 45 to mass 44) was compared with a reference gas.
Calculations
Energy expenditure and whole body fatty acid oxidation.
Resting metabolic rate (RMR) was calculated from resting V̇o2 and V̇co2 measurements using the Weir equation (21). To estimate total daily energy expenditure during CON (i.e., no exercise) we multiplied the RMR measured on day 1 by a “physical activity level” (PAL) factor of 1.4, which is representative of relatively sedentary individuals (3). To calculate total daily energy expenditure during the exercise trials, we added the energy expenditure measured during exercise to the estimated energy expenditure during the hours not exercising, which we estimated by multiplying RMR by a PAL value of 1.5 (a slightly higher PAL value was used during the exercise trials to capture the relatively small elevation in energy expenditure in the hours after exercise). Whole body fat/triacylglycerol oxidation (g/min) was calculated from V̇o2 and V̇co2 measurements using the equations of Frayn (9). Whole body fatty acid oxidation was calculated by dividing triacylglycerol oxidation by an estimated molecular weight of triacylglycerol (860 g/mol) and multiplying by 3.
Insulin sensitivity.
Plasma glucose and insulin concentration measured during the IVGTT were used to calculate the insulin sensitivity index (Si), using the MINMOD Millennium computer analysis software (version 6.02) (2).
Plasma palmitate rate of appearance and disappearance.
Steady-state fatty acid concentration and TTR were achieved during isotope infusion; therefore palmitate rate of appearance into plasma (Ra) = rate of disappearance from plasma (Rd) and could be calculated using Steele's equation for steady-state conditions (31).
Plasma palmitate oxidation.
Plasma palmitate oxidation was calculated as: plasma palmitate oxidation = (Eco2 × V̇co2)/(TTRp × AR), where Eco2 is the 13C enrichment in breath CO2, TTRp is the steady-state TTR in plasma, and AR is the acetate carbon recovery factor, calculated as previously described (30). Percent palmitate Rd oxidized was calculated as the ratio of plasma palmitate oxidation to plasma palmitate Rd.
Statistical Analysis
A one-way ANOVA with repeated measures and, when appropriate, Tukey's post hoc pairwise comparisons were used to test for significant differences in factor means. Statistical significance was set at the P ≤ 0.05 level. All data are presented as means ± SE.
RESULTS
Energy Expenditure, Intake, and Balance
Resting energy expenditure measured on day 1 (Table 2) and day 2 (data not shown) was nearly identical among trials. Exercise energy expenditure (Table 2) and respiratory exchange ratio during exercise (range: 0.86–0.87) were also nearly identical among the three exercise trials. Therefore, as designed, estimated total energy expenditure was not different among the exercise trials but was significantly elevated compared with the sedentary CON trial. Also by design, energy intake was lower during CON and LOW-EN compared with BAL and LOW-CHO (Table 2). Accordingly, subjects remained in energy balance during all trials with the exception of LOW-EN, when subjects were exposed to ∼750 kcal energy deficit.
Table 2.
Estimated energy balance during the trials (kcal/day)
| CON | BAL | LOW-EN | LOW-CHO | |
|---|---|---|---|---|
| Resting energy expenditure | 1,582 ± 40 | 1,557 ± 36 | 1,543 ± 34 | 1,576 ± 53 |
| Exercise energy expenditure | NA | 817 ± 36 | 825 ± 32 | 804 ± 34 |
| Total energy expenditure | 2,274 ± 76a | 3,005 ± 104b | 3,022 ± 109b | 3,079 ± 103b |
| Total intake | 2,250 ± 78a | 3,138 ± 107b | 2,268 ± 90a | 3,091 ± 110b |
| Energy balance | −23 ± 60a | 133 ± 89a | −755 ± 85b | 12 ± 80a |
Values are means ± SE. Total energy expenditure estimated as: (1.4-1.5 × resting energy expenditure) + exercise energy expenditure. Values with nonmatching letters in each row are significantly different (P < 0.05). NA, not applicable.
Insulin Sensitivity
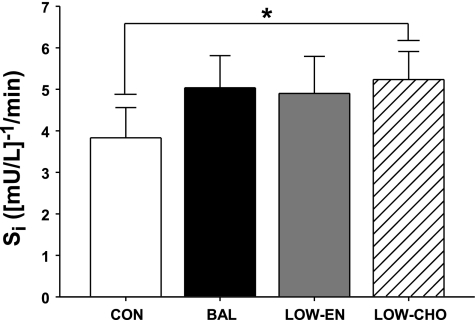
In general, insulin sensitivity tended to be elevated the day after all of the exercise trials compared with control (P = 0.03–0.22; Fig. 2). However, this difference only reached statistical significance during LOW-CHO vs. CON (P = 0.03), even though subjects were in energy balance during both of these trials. Conversely, despite eating several hundred fewer kilocalories during LOW-EN compared with BAL, Si was identical during these trials (Fig. 2).
Fig. 2.
Insulin sensitivity index (Si) measured using an IVGTT the morning after exercise. *P < 0.05, low carbohydrate intake (LOW-CHO) vs. control trial (CON). LOW-EN, low energy intake; BAL, subjects in nutrient balance.
Skeletal Muscle Substrate Stores
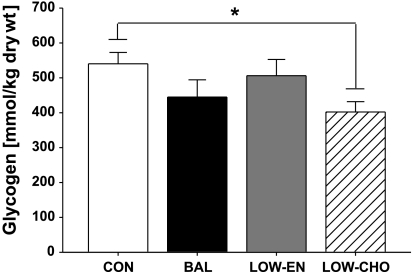
As expected, low carbohydrate intake after exercise during LOW-CHO resulted in a ∼25% lower muscle glycogen concentration compared with CON (P < 0.01, Fig. 3). In contrast, the relatively high carbohydrate intake after exercise during BAL and LOW-EN replenished muscle glycogen concentration to levels similar to that found when they did not exercise (CON). IMTG concentration was similar during CON, BAL, LOW-EN, and LOW-CHO (34.4 ± 8.4, 37.7 ± 5.4, 30.6 ± 5.4, and 31.8 ± 6.2 mmol/kg dry weight, respectively; P = 0.8).
Fig. 3.
Skeletal muscle glycogen concentration measured in muscle biopsy samples collected the morning after exercise. *P < 0.05, LOW-CHO vs. CON.
Fatty acid (Palmitate) Mobilization and Oxidation
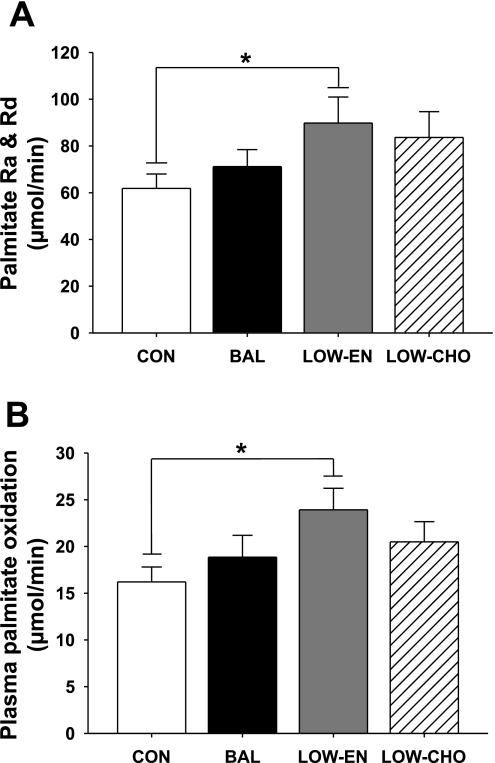
Negative energy balance after exercise (LOW-EN) increased palmitate Ra and Rd the next morning by 45% compared with CON (P = 0.04, Fig. 4A). In parallel, plasma palmitate oxidation was also greater during LOW-EN compared with CON (Fig. 4B). Palmitate Ra and Rd were ∼35% greater during LOW-CHO compared with CON, but this difference did not reach statistical significance (P = 0.11). Plasma palmitate oxidation during LOW-CHO was also not significantly different from CON (Fig. 4B). An exercise-induced increase in palmitate Ra and Rd was not observed when subjects were in both energy and carbohydrate balance (BAL). Whole body fatty acid oxidation rates were similar during CON, BAL, LOW-EN, and LOW-CHO (297 ± 24, 261 ± 32, 269 ± 23, 301 ± 30 μmol/min, respectively; P = 0.22), and the percentage of plasma palmitate uptake that was oxidized was also not different among trials (range: 26–29%; P = 0.51).
Fig. 4.
Palmitate rate of appearance into plasma (Ra) and rate of disappearance from plasma (Rd) (A), and plasma palmitate oxidation (B) the morning after exercise. *P < 0.05, LOW-EN vs. CON.
Plasma Substrate and Insulin Concentrations
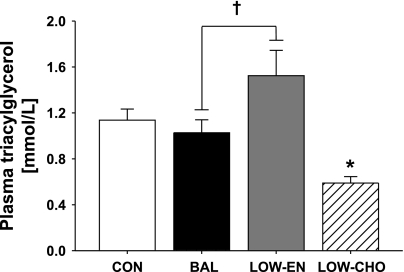
The morning after exercise, overnight fasted plasma concentrations of glucose, fatty acid, and insulin were not different among trials (Table 3). Similarly, exercise had no effect on plasma triacylglycerol concentrations the next morning when subjects were in carbohydrate and energy balance (BAL) (Fig. 5). However, plasma triacylglycerol concentration was significantly lower the morning after exercise during LOW-CHO compared with all other trials. Interestingly, plasma triacylglycerol concentration the morning after exercise during LOW-EN was significantly greater than BAL (P = 0.02; Fig. 5) despite consuming less dietary fat and the same amount of carbohydrate in the meals after exercise.
Table 3.
Plasma substrate and insulin concentrations
| CON | BAL | LOW-EN | LOW-CHO | |
|---|---|---|---|---|
| Glucose, mmol/l | 5.4 ± 0.2 | 5.0 ± 0.2 | 4.9 ± 0.1 | 5.0 ± 0.1 |
| Fatty acid, mmol/l | 0.34 ± 0.07 | 0.31 ± 0.05 | 0.45 ± 0.07 | 0.35 ± 0.06 |
| Insulin, μU/ml | 12.0 ± 1.0 | 10.5 ± 1.0 | 11.9 ± 1.2 | 9.3 ± 0.9 |
Values are means ± SE.
Fig. 5.
Plasma triacylglycerol concentration measured at 0800 the morning after exercise. †P < 0.05 LOW-EN vs. BAL. *P < 0.05, LOW-CHO vs. all trials.
DISCUSSION
The primary aim of this study was to compare the effects of an exercise-induced energy deficit vs. carbohydrate deficit on insulin sensitivity and lipid metabolism. Our findings demonstrate that maintaining an energy deficit after a single session of exercise did not augment the effect of exercise on insulin sensitivity. Additionally, our study confirms previous work demonstrating that consuming low carbohydrate content in meals after exercise prevents the restoration of muscle glycogen concentration the next morning and significantly increased insulin sensitivity the next day. Importantly, in our study we demonstrated that this exercise-induced improvement in insulin sensitivity occurred despite maintaining energy balance. Finally, we found that the exercise-induced energy deficit provided the most potent effect on endogenous lipid metabolism as measured by an elevated plasma triacylglycerol concentration, as well as an increased plasma fatty acid mobilization and oxidation the day after exercise.
Our findings are in line with previous studies indicating the insulin-sensitizing effects of exercise are enhanced when the carbohydrate content in meals ingested after exercise is kept relatively low, and muscle glycogen concentration is prevented from becoming fully restored (4, 5, 19). Additionally, the magnitude of change in insulin action the day after exercise was recently shown to be directly proportional to the magnitude of the carbohydrate deficit (17). In contrast to our findings, Holtz et al. (17) recently reported that maintaining a carbohydrate deficit after exercise did not significantly increase insulin-mediated glucose disposal when subjects were in energy balance. Perhaps the discrepancy between our two studies can be explained by the greater carbohydrate restriction we imposed in our subjects compared with Holtz et al. (250 vs. 60 g less carbohydrate consumed during carbohydrate restriction, respectively). However, we must acknowledge the trend we observed for an increase in insulin sensitivity the day after all of our exercise trials compared with no exercise. In our study, we provided adequate carbohydrate during BAL and LOW-EN to restore muscle glycogen back to levels found without exercise (CON). However, insulin sensitivity may continue to be elevated until glycogen concentration is raised to levels above that measured before exercise (i.e., glycogen supercompensation) (5, 19). Therefore, even when carbohydrate intake after exercise is adequate to restore muscle glycogen to preexercise levels, exercise, per se, may still slightly improve insulin sensitivity until muscle glycogen supercompensation is achieved. This may explain the trend for enhanced insulin sensitivity we observed in all of our exercise trials compared with CON. Most notably, in addressing the issue of an exercise-induced energy deficit independent of carbohydrate deficit, we fed subjects exactly the same carbohydrate content after exercise during LOW-EN and BAL and found that despite ingesting several hundred fewer total kilocalories during LOW-EN compared with BAL (thereby establishing a marked negative energy balance during LOW-EN), insulin sensitivity was identical the day after exercise during these trials. We interpret this to suggest that there was no added insulin-sensitizing effect of an energy deficit.
Although maintaining an energy deficit after exercise did not contribute to enhanced insulin sensitivity the next day, energy deficit did augment fatty acid Ra and Rd. Importantly, the increase in fatty acid uptake was paralleled by an increase in plasma fatty acid oxidation. However, the magnitude of the increase in plasma fatty acid oxidation during LOW-EN did not quantitatively match the increase in fatty acid Rd, indicating that not all of the excess fatty acid delivery was oxidized. In an effort to determine other possible “fates” of the remaining increase in fatty acid flux, we determined that IMTG concentration was not elevated during LOW-EN, suggesting that these fatty acids were not being esterified into triacylglycerides in skeletal muscle. Conversely, the elevated plasma triacylglyceride concentration during LOW-EN indicates some of these “excess” fatty acids were likely esterified in the liver and released as triacylglyceride within very low-density lipoproteins (VLDLs).
One session of exercise has been reported to potently suppress postprandial triacylglycerol concentrations the day after exercise (1, 32, 33), perhaps largely due to an exercise-mediated increase in lipoprotein lipase activity (11, 16). However, the effect of prior exercise on the fasting plasma triacylglycerol concentration is less clear, with some studies reporting lower fasting plasma triacylglycerol concentrations the morning after exercise (1, 32, 33), while others report no effect of prior exercise (12, 14). Using the data from our BAL trial to assess the effect of exercise on fasting plasma triacylglycerol concentration without the confounding influences of an energy deficit and/or carbohydrate deficit, our findings suggest that exercise, per se, did not alter plasma triglyceride concentration. Similar to these findings, a very recent study reported that >90 min of moderate- to high-intensity exercise also did not affect fasting plasma triacylglycerol concentrations the next morning when subjects were refed adequate carbohydrate and total energy in the hours after exercise (14). In contrast, we found that altering either the energy or carbohydrate content of the meals after exercise affected fasting plasma triacylglycerol concentration the next morning. As addressed above, an energy deficit resulted in an augmented fasting plasma triacylglycerol concentration, perhaps due to the elevated fatty acid mobilization found with an energy deficit. Conversely, inadequate dietary carbohydrate after exercise (LOW-CHO) resulted in a suppression of plasma triacylglycerol concentration the next morning. Although it may seem counterintuitive, high-carbohydrate diets are often found to elevate plasma triacylglycerol concentration (8, 13), in part due to an accelerated hepatic VLDL-triacylglycerol secretion (23). Therefore, it is not surprising that our low carbohydrate intake after exercise during LOW-CHO suppressed plasma triacylglycerol concentration.
Attempting to examine the independent effects of energy deficit and carbohydrate deficit presents several challenges and limitations. For example, because energy deficit during our LOW-EN trial was achieved by reducing fat intake only, an alternative interpretation may be that these findings are the direct result of lowering dietary fat intake, and not due to an energy deficit, per se. Similarly, because we increased dietary fat intake during LOW-CHO to compensate for the reduction in carbohydrate calories, it is possible that the observed effects during LOW-CHO were a consequence of the increased fat intake. However, while the human body is keenly sensitive to even small changes in dietary carbohydrate (largely through the subsequent increase in insulin), evidence suggests that this is not the case for acute changes in dietary fat availability. Additionally, we acknowledge that the observed trend for an increase in Si during BAL and LOW-EN compared with CON, as well as the trend for increased palmitate Ra and Rd during LOW-CHO compared with CON, may not have reached statistical significance due to the relatively low statistical power stemming from enrolling only nine subjects in this study. A power analysis indicates that for 80% power we would need to recruit at least another eight subjects (n = 17) in order for the differences observed between these trials to reach statistical significance at an α-level of 0.05. Furthermore, much of our interpretation about our exercise and diet treatments on insulin sensitivity is focused on potential effects within skeletal muscle. Although we contend that most of the exercise-induced adaptations will occur within the muscles that were activated during exercise, we acknowledge that changes in hepatic glucose metabolism could also impact our measurement of whole body insulin sensitivity (Si). Finally, because this study was conducted in healthy nonobese male subjects, the findings may not necessarily translate to other populations. This healthy subject population was studied so that we could better isolate the effects of postexercise macronutrient intake on insulin sensitivity without the confounding influence of cardiovascular or metabolic complications. Follow-up studies will be designed to specifically investigate whether these findings are consistent in an obese, insulin-resistant population, and future studies must also be designed to determine whether there is a sex difference in these outcomes.
It is well-recognized that exercise is a key component of a healthy lifestyle, and increasing evidence clearly indicates that profound metabolic health improvements occur even after a single session of exercise. Moreover, the foods eaten after exercise can greatly impact the metabolic responses for several hours and even days after the exercise session. Our findings demonstrate that maintaining an energy deficit after exercise does not contribute to the exercise-induced enhancement of insulin sensitivity when carbohydrate in meals after exercise is adequate to replenish muscle glycogen concentration. Additionally our findings confirm the important role of dietary carbohydrate after exercise in regulating insulin sensitivity the next day. However, maintaining an energy deficit after exercise did alter lipid metabolism, as noted by an increased rate of fatty acid mobilization in the circulation and an elevated fasting plasma triacylglyceride concentration the next morning. Together these findings underscore the importance of carefully controlling for both macronutrient and energy intake when attempting to assess changes in insulin sensitivity and lipid metabolism after exercise.
GRANTS
This study was supported by National Institutes of Health Grants NIH-NIDDK-5P60DK-20572 (to the MDRTC) and NIH-UL1-RR-024986 (to the MCRU).
DISCLOSURES
None of the authors had any conflict of interest pertaining to the topic or contents of this article.
ACKNOWLEDGMENTS
We thank the staff of the Michigan Clinical Research Unit (MCRU) and the Michigan Metabolomics Obesity Center (MMOC) for help conducting the experimental protocols; Lisa Michael for recruiting, screening, and enrolling subjects, and for help with study diets; Alexander Hinko, Ph.D. for technical support with the GC/MS; staff in the Michigan Diabetes Research and Training Center (MDRTC) chemistry core laboratory for assistance with the insulin assay; Metabolic Solutions for assistance with breath sample analysis; and the subjects for their enthusiastic participation.
REFERENCES
- 1.Aldred HE, Perry IC, Hardman AE. The effect of a single bout of brisk walking on postprandial lipemia in normolipidemic young adults. Metabolism 43: 836–841, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 79: 790–800, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 50: 72–92, 1996 [PubMed] [Google Scholar]
- 4.Bogardus C, Thuillez P, Ravussin E, Vasquez B, Narimiga M, Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest 72: 1605–1610, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Dela F, Mikines KJ, von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol Endocrinol Metab 263: E1134–E1143, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol 291: R1120–R1128, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Farquhar JW, Frank A, Gross RC, Reaven GM. Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest 45: 1648–1656, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 21: 139–144, 1980 [PubMed] [Google Scholar]
- 11.Gill JM, Herd SL, Vora V, Hardman AE. Effects of a brisk walk on lipoprotein lipase activity and plasma triglyceride concentrations in the fasted and postprandial states. Eur J Appl Physiol 89: 184–190, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gill JM, Murphy MH, Hardman AE. Postprandial lipemia: effects of intermittent versus continuous exercise. Med Sci Sports Exerc 30: 1515–1520, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg H, Olefsky JM, Kimmerling G, Crapo P, Reaven GM. Induction of hypertriglyceridemia by a low-fat diet. J Clin Endocrinol Metab 42: 729–735, 1976 [DOI] [PubMed] [Google Scholar]
- 14.Harrison M, O'Gorman DJ, McCaffrey N, Hamilton MT, Zderic TW, Carson BP, Moyna NM. Influence of acute exercise with and without carbohydrate replacement on postprandial lipid metabolism. J Appl Physiol 106: 943–949, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55: 512–517, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Herd SL, Kiens B, Boobis LH, Hardman AE. Moderate exercise, postprandial lipemia, and skeletal muscle lipoprotein lipase activity. Metabolism 50: 756–762, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Holtz KA, Stephens BR, Sharoff CG, Chipkin SR, Braun B. The effect of carbohydrate availability following exercise on whole-body insulin action. Appl Physiol Nutr Metab 33: 946–956, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism 40: 406–409, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Kawanaka K, Han DH, Nolte LA, Hansen PA, Nakatani A, Holloszy JO. Decreased insulin-stimulated GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Am J Physiol Endocrinol Metab 276: E907–E912, 1999 [DOI] [PubMed] [Google Scholar]
- 20.King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol 64: 1942–1946, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Mansell PI, Macdonald IA. Reappraisal of the Weir equation for calculation of metabolic rate. Am J Physiol Regul Integr Comp Physiol 258: R1347–R1354, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab 254: E248–E259, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr 73: 892–899, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60: 405–412, 1974 [DOI] [PubMed] [Google Scholar]
- 25.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78: 1568–1578, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol 66: 876–885, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Robertson MD, Henderson RA, Vist GE, Rumsey RD. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am J Clin Nutr 75: 505–510, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am J Physiol Endocrinol Metab 273: E623–E629, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol Endocrinol Metab 269: E649–E656, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 32.Tsetsonis NV, Hardman AE. Effects of low and moderate intensity treadmill walking on postprandial lipaemia in healthy young adults. Eur J Appl Physiol Occup Physiol 73: 419–426, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Tsetsonis NV, Hardman AE. Reduction in postprandial lipemia after walking: influence of exercise intensity. Med Sci Sports Exerc 28: 1235–1242, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Votruba SB, Atkinson RL, Hirvonen MD, Schoeller DA. Prior exercise increases subsequent utilization of dietary fat. Med Sci Sports Exerc 34: 1757–1765, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49: 325–331, 2000 [DOI] [PubMed] [Google Scholar]