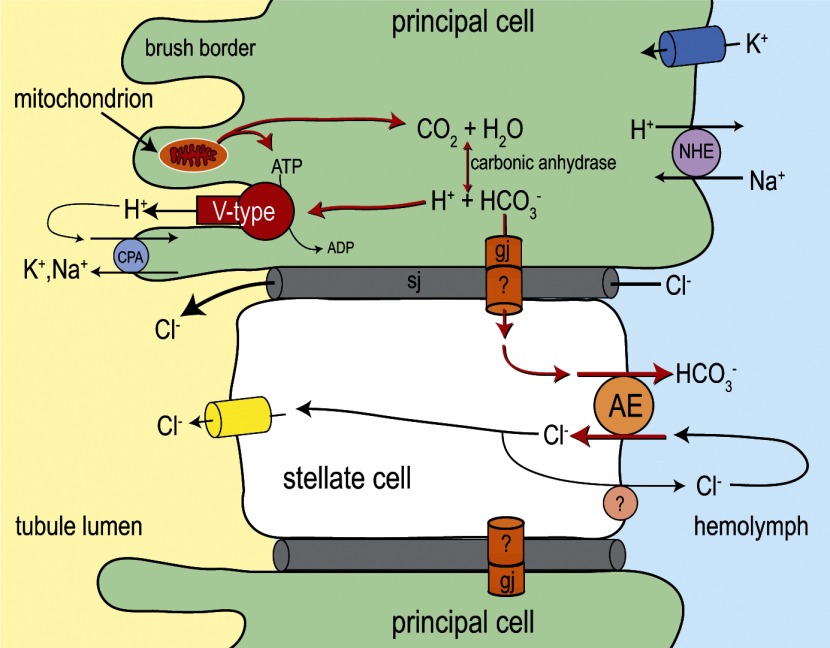
Fig. 14.
Hypothesized metabolic model of stellate cell function in Aedes Malpighian tubules. The illustration shows a stellate cell intercalated between 2 principal cells. As emphasized by the red arrows, we propose that AeAE contributes primarily to the handling of intracellular HCO3− that is generated by the mitochondrial metabolism of principal cells during diuretic conditions. AeAE also may contribute to a transcellular pathway for Cl− secretion, but this is considered a minor role (see text for details). A recycling mechanism for Cl− across the basal membrane of stellate cells cannot be ruled out. Principal and stellate cells are shown to be connected via 1) paracellular septate junctions (sj), which provide the primary pathway for transepithelial Cl− secretion (3), and 2) intercellular gap junctions (gj), which provide a putative pathway for the transport of HCO3− to stellate cells. Previous studies in mosquito Malpighian tubules have shown evidence of an apical V-type H+-ATPase in the brush border of principal cells (52, 86), a carbonic anhydrase in principal cells (67), gap junctions in principal cells (87), basal K+ channels in principal cells (6), a basal Na/H exchanger (NHE) in principal cells (53, 57), and apical Cl− channels in stellate cells (45). The molecular identity of the apical cation/H+ antiporter (CPA) has yet to be identified (56). See Ref. 7 for a detailed review of the transepithelial transport mechanisms in mosquito Malpighian tubules.