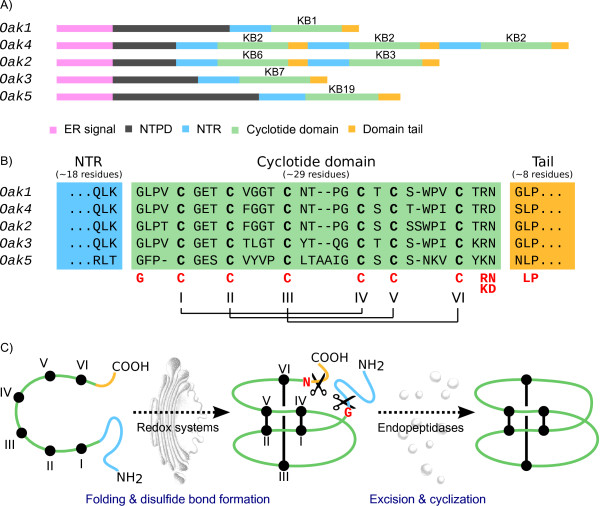
Figure 3.
Cyclotide precursor constructions and maturation in O. affinis. A) Cyclotide precursor constructions of O. affinis. (ER, endoplasmic reticulum; NTPD, N-terminal pro-domain; NTR, N-terminal repeat; "kB" is the abbreviation of kalatas). B) Alignment of cyclotide domains including their flanking sequences. Red letters under the sequences indicate conserved or relatively conserved amino acids. The six conserved cysteines are numbered using Roman numerals and their connectivity is shown. C) Proposed molecular model for in vivo formation of cyclotides in O. affinis. Formation of disulfide bond requires redox systems, which consists of the ferredoxin/thioredoxin system, the NADP/thioredoxin system and the glutathione/glutaredoxin system. Excision of the cyclotide domains from precursor proteins and their cyclisation are thought to be catalysed by endopeptidases [18,19]. Round black disks represent the cysteins and they are connected by disulfide bonds showing in the structures.