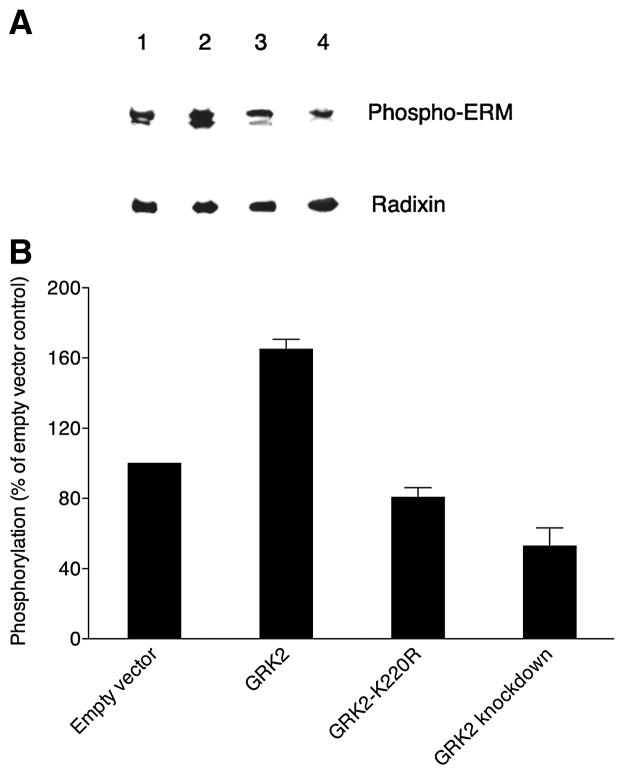
Figure 7. GRK2-dependent phosphorylation of ERM proteins in MDCK cells.
(A) A representative Western blot probed with phospho-ERM antibody is shown at the top for extracts from confluent MDCK cell cultures stably expressing radixin-HA that were then transiently transfected with empty vector (lane 1), wild-type GRK2 (lane 2), kinase-dead GRK2-K220R mutant (lane 3) or GRK2-specific shRNA (GRK2 knockdown; lane 4). The same blot stripped and reprobed with anti-radixin antibody is shown at the bottom. (B) Levels (mean and SEM) of phosphorylated ERM proteins are shown, derived from band intensities on Western blots for three separate experiments as in A and expressed as a percent of the level for empty vector controls (normalization to the parallel control for each experiment prior to statistics).