Abstract
Rhabdomyosarcoma (RMS), the most common soft-tissue sarcoma in children, is cured with conventional therapy in 70%. However, 5 year survival for those who relapse is about 30% and drops to about 15% for those with unfavorable histologies (alveolar/undifferentiated subtypes). We describe outcomes of 62 subjects receiving autologous blood/bone marrow transplants for RMS between 1989 and 2003 and reported to CIBMTR. Histological subtype was confirmed by reviewing pathology reports. Transplant-related mortality (TRM), progression-free survival (PFS) and survival were evaluated. Overall 73% of subjects were < 20 years; 39% had cancer bulk >5cm, 63% had metastasis at diagnosis, 55% had unfavorable histologies, 92% had cancer responsive to chemotherapy pretransplant and 67% were in 1st remission. The 1-year TRM was 5% (95% CI, 1–12%) and the 5 year PFS and survival were 29% (95% CI, 18–41%) and 32% (95% CI, 21–44%) respectively. There was only a 4% relapse rate after the first year. There were no differences in 5 year PFS or survival based on histological subtype, transplant in 1st remission vs. relapse (36% vs. 29%; p=0.5), or transplantation for poor-risk histologies in 1st remission vs. relapse (34% vs. 33%; p=0.9). Our data indicate that autotransplants for RMS disease are typically done in patients with disease responsive to chemotherapy pretransplant, with approximately one-third long-term survivors. Despite high risk factors, we also found a low TRM, perhaps reflecting the migration from marrow to blood stem cells as the graft source. Even when performed after relapse for alveolar/undifferentiated histologies, long-term survivals were seen seemingly better than results with conventional therapies.
Keywords: Rhabdomyosarcoma, autotransplant, soft-tissue sarcoma
INTRODUCTION
In Rhabdomyosarcoma (RMS), the cures have increased from 25–70% when combinations of surgery, intensive combination chemotherapy and radiation therapy are used based on well-described prognostic factors(1). Adverse prognostic factors, which correlate with decreased survival include: larger size (>10cm), non-bladder, prostate, extremity or meningeal primary sites, alveolar or undifferentiated histologies, and residual disease after the initial surgery(1–4). These parameters dictate therapy and predict outcome. Subjects with favorable histologies without metastases have 90% cures whereas subjects with unfavorable histologies and metastases have only 25% cures. In addition, approximately 1/3 who initially respond, will relapse and up to 90% of these ultimately die of disease-progression.
Based on the initial sensitivity of RMS to chemotherapy and on the fact that some persons are cured with conventional therapies, several groups have studied the use of blood and bone marrow transplants(5–9). The procedure is safe but little can be concluded about its efficacy because of the number of transplants reported and the lack of appropriate controls and randomized trials.
The Center for International Blood and Marrow Transplantation Research (CIBMTR) has data on a relatively large number of transplants. Centers who transplanted subjects for this diagnosis were invited to submit detailed demographic, treatment and follow-up data on their subjects. In this descriptive analysis, we evaluated treatment-related mortality (TRM) and survival of the various prognostic groups focusing on remission state at transplant and favorable vs. unfavorable histological cohorts.
PATIENTS AND METHODS
Data Sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) established in 2004 that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic SCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Subjects are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, sex, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. All CIBMTR teams contribute TED data. More detailed disease, pre- and post-transplant clinical information are collected on a subset of registered subjects selected for CRF data by a weighted randomization scheme. TED and CRF level data are collected pre-transplant, 100 days and six months post transplant and annually thereafter or until death.
Patients
Between 1989 and 2002, 110 subjects with RMS who received a first autologous marrow and/or peripheral blood stem cell rescue were registered with the CIBMTR. Comprehensive clinical data about the disease and transplant sufficient for analysis was available for 78 of these subjects. The 32 remaining subjects had only registration level data. Detailed report forms for the 32 subjects that were missing relevant clinical data on disease and transplant characteristics as well as pathology report forms for all the subjects were requested from teams so as to further confirm the disease diagnosis. We received pathology report forms and complete clinical data for only 67 subjects. The transplant teams for the remaining 43 subjects indicated that the pathology reports were unavailable to review or did not respond to our multiple requests for data. We carefully reviewed the pathology report for each of the 67 subjects to confirm their diagnosis and to determine their histological subgroup. After further review of the pathology reports, we excluded 5 subjects (histology: non-rhabdomyosarcoma) from our patient population. We defined poor risk histology (n=34) as an alveolar or undifferentiated subtype, whereas patients with good risk histology (n=21) had embryonal or botryoid subtypes. A total of 62 confirmed subjects from 30 transplant centers, were used in all of our descriptive analyses.
Study Endpoints
The goal of this study was to describe the clinical outcomes in subjects with RMS after an autologous bone marrow or blood cell transplant. The primary endpoints included: treatment-related mortality (TRM), progression/relapse, progression-free survival (PFS), overall survival (OS), neutrophil and platelet recovery. TRM was defined as death within 28 days post-transplant or death without disease progression after 28 days post transplant. Subjects with disease progression were censored at the time of progression with progression/relapse as the competing risk.
Progression/relapse was defined as progressive disease post-transplant (≥28 days) or recurrence. It could follow a period of “stable” disease post-transplant, or a partial or complete remission. Progression/relapse represents new or larger areas of disease (≥25% increase in largest diameter) compared to the best post-transplant disease state. Progression/relapse was summarized by the cumulative incidence function estimate with TRM as the competing risk. For analysis of PFS, subjects were considered treatment-failures at the time of disease progression or death from any cause. Subjects alive without evidence of RMS-progression were censored at last follow-up. For analysis of OS, failure was defined as death from any cause and alive subjects were censored at time of last follow-up.
Statistical Analysis
Patient-, disease-, and transplant-related variables are described for all subjects in Table 1. Probabilities of TRM, relapse/progression, neutrophil and platelet engraftment were calculated using cumulative incidence estimates(10, 11). The cumulative incidence calculated for neutrophil and platelet engraftment treated death as a competing risk whereas in the cumulative incidence calculated for TRM, relapse/progression was treated as the competing risk. Univariate probabilities of progression-free survival and overall survival were calculated using the Kaplan-Meier estimator(12). The Log rank test was used for comparing survival curves. We also compared outcomes between subjects who were transplanted in 1st remission vs. all others; subjects presenting with metastatic disease vs. those without metastasis at diagnosis and those in the two histological groups in 1st remission vs. those who had relapsed. Comparisons between these groups used chi-square statistic for categorical variables and the Kruskal-Wallis test for continuous variables.
Table 1.
Characteristics of subjects who underwent an autologous bone marrow or peripheral blood transplant for Rhabdomysarcoma between 1989 and 2003, reported to the CIBMTR.
| Patient characteristics | N (%) |
|---|---|
| Patient-related: | |
| Number of subjects | 62 |
| Number of centers | 30 |
| Age, median (range), years | 14 (3–40) |
| 0–9 | 28 (45) |
| 10–19 | 17 (28) |
| 20–29 | 12 (19) |
| 30–49 | 5 (8) |
| Male sex | 32 (52) |
| KPS at transplant ≥90% | 48 (77) |
| Disease-related: | |
| Tumor burden, > 5 cm | 24 (69) |
| Primary site of tumor | |
| Extremity/limb girdle | 20 (35) |
| Head and neck | 21 (37) |
| Truncal | 2 9 4) |
| Visceral/retropreritoneal | 12 (21) |
| Other | 2 (4) |
| Metastases at diagnosis? | 39 (67) |
| Metastatic sites at diagnosis | |
| BM ± other (no CNS) | 15 (38) |
| Lungs ± other (no CNS) | 14 (36) |
| Liver ± other | 1 (3) |
| CNS only | 1 (3) |
| Other | 8 (21) |
| No relapse prior to transplant | 39 (67) |
| No | |
| Yes | 19 (33) |
| ≥12months initial diagnosis to 1st recurrence/progression, months | 12 (67) |
| Initial response to chemotherapy | |
| Complete response | 25 (45) |
| Partial response | 18 (33) |
| Stable disease/Progressive disease | 6 (11) |
| Not evaluable | 6 (11) |
| Lines of Chemotherapy | |
| 1 | 32 (52) |
| 2 | 20 (32) |
| 3+ | 9 (15) |
| Disease status prior to conditioning | |
| Complete response | 36 (61) |
| Partial response | 18 (31) |
| Stable disease/Progressive disease | 5 (8) |
| Histology | |
| Alveolar | 31 (50) |
| Embryonal | 20 (32) |
| Botryoid | 1 (2) |
| NA-NOS | 7 (11) |
| Undifferentiated | 3 (5) |
| Transplant-related: | |
| Time from initial diagnosis to transplant median (range), months | 10 (3–83) |
| <12months | 39 (63) |
| ≥12 months | 23 (37) |
| Conditioning regimen | |
| Etopside + Cy + LPAM ± other | 6 (10) |
| Etopside + Cy ± other | 19 (31) |
| Etopside + LPAM ± other | 15 (24) |
| Etopside + Carb ± other | 1 (2) |
| LPAM ± other | 12 (19) |
| Cy ± other | 6 (10) |
| Othera | 3 (5) |
| Source of stem cells | |
| Bone marrow | 13 (21) |
| Peripheral blood | 43 (69) |
| Both | 6 (10) |
| Growth factor post-transplantb | 50 (81) |
| Planned radiation treatment given post-transplant | 15 (25) |
| Year of transplant | |
| 1989–1991 | 1 (2) |
| 1992–1994 | 8 (13) |
| 1995–1997 | 33 (53) |
| 1998–2000 | 18 (29) |
| 2001–2003 | 2 (3) |
| Median follow-up of survivors, median (range), months | 78 (22–172) |
| Causes of death | |
| Primary cancer | 39 (91) |
| Interstitial pneumonitis | 2 (5) |
| Organ toxicity | 1 (2) |
| Other cancer | 1 (2) |
Abbreviations: PB= peripheral blood; CNS = central nervous system; CR= complete remission; TBI= total body irradiation; Cy= cyclophosphamide; LPAM=melphalan; Carb= carboplatin; IPN = interstitial pnuemoniatis; KPS=karnofsky score at transplant; BM=bone marrow.
Other conditioning regimen (n=3): Carboplatin only (n=2); Mitoxantrone + Paclitaxel + Thiotepa (n=1).
Growth factor, GCSF or GMCF, was delivered to promote engraftment. This was initiated between day -1 and day 7.
RESULTS
Patient Demographics
At the time of transplantation, the median age was 13 years, but 17% were older than 19 years. Of the entire cohort, 35 % had limb/girdle and 37% head and neck primaries. Tumor diameter > 5 cm at time of disease diagnosis was found in 69% and metastatic disease was seen in 67%. The most common sites of metastasis were bone marrow (38%) and lung (36%). Pathology subtypes included: alveolar (50%) and embryonal (32%). One patient had botryoid histology and three had undifferentiated tumors. While 78% had chemotherapy responsive disease after induction therapy, only 25 (45%) of evaluable subjects were in CR. All but one patient received involved field radiotherapy as part of initial therapy. Of the 62 subjects 61% were either in a 1st (52%) or subsequent CR. One third of subjects were transplanted after relapse. Overall 92% were transplanted for disease responding to chemotherapy with a third after two regimens and 15% after three chemotherapy regimens. The median time from diagnosis to transplant was 10 months. The conditioning regimens were varied but included melphalan in 44% and etoposide in 84%. Consistent with the timing of this analysis, 79% received peripheral blood stem cells with (10%) or without bone marrow stem cell (69%) as the transplant source.
Risk Factors at Transplant: Remission Status and Histology
There were no initial demographic differences for those transplanted as consolidation of 1st remission (n=38) vs. those transplanted after relapse (n=21), including, age at diagnosis, primary site, histology, presence of metastatic disease at transplant or response to initial therapy (Table 2). However, a difference was seen in the percent of subjects transplanted in CR with 53% vs.73% for the 1st remission vs. relapse groups respectively (p =0.032), perhaps suggesting a selection bias for transplanting only subjects whose cancer is responsive to chemotherapy once they had relapsed. For those with good risk (n=21) vs. poor risk histology (n=34), there were no differences in their demographics (Table 3), although trends were seen for those with poor risk histology who had a higher frequency of marrow involved at diagnosis (44 vs.. 14%; p=0.244), a CR to primary therapy (53% vs. 26%; p=0.115) and being in a CR at transplant (70% vs.. 48%; p=0.247).
Table 2.
Patient characteristic comparison between those subjects in 1st remission prior to transplant versus subjects who relapsed prior to transplant before receiving an autologous bone marrow or peripheral blood transplant for Rhabdomysarcoma between 1989 and 2003. All transplants were reported to the CIBMTR.
| 1st remission (N=38) | Relapse (N=21) | ||||
|---|---|---|---|---|---|
| N eval | N (%) | N eval | N (%) | P- valuea | |
| Number of subjects | 38 | 21 | |||
| Age, <20 years | 38 | 24 (63) | 21 | 18 (86) | 0.263 |
| KPS ≥90 | 38 | 32 (84) | 21 | 15 (71) | 0.242 |
| Tumor burden, > 5 cm | 21 | 15 (71) | 13 | 8 (62) | 0.735 |
| Primary site of tumor | 36 | 19 | *** | ||
| Extremity/limb girdle | 12 (33) | 7 (37) | |||
| Head and neck | 14 (39) | 7 (37) | |||
| Truncal | 2 (6) | -- | |||
| Visceral/retropreritoneal | 7 (19) | 4 (21) | |||
| Other | 1 (3) | 1 (5) | |||
| Metastases at diagnosis | 37 | 30 (81) | 19 | 7 (37) | 0.002 |
| Metastatic sites at diagnosis | 38 | 7 | *** | ||
| BM ± other (no CNS) | 12 (39) | 2 (29) | |||
| Lungs ± other (no CNS) | 12 (39) | 1 (14) | |||
| Liver ± other | -- | 1 (14) | |||
| CNS only | 1 (3) | -- | |||
| Other | 5 (16) | 3 (44) | |||
| Missing | 1 (3) | 2 (29) | |||
| Response to 1st line of therapy | 35 | 20 | |||
| Complete response | 14 (40) | 11 (55) | |||
| Partial response | 16 (46) | 2 (10) | |||
| Stable disease/Progressive disease | 1 (3) | 5 (25) | |||
| Not evaluable | 4 (11) | 2 (10) | |||
| Disease status prior to conditioning | 38 | 20 | 0.032 | ||
| Complete response | 20 (53) | 15 (75) | |||
| Partial response | 16 (42) | 2 (10) | |||
| Stable disease/Progressive disease | 2 (5) | 3 (15) | |||
Abbreviations: CNS = central nervous system; KPS=Karnofsky score at transplant; BM=bone marrow.
Chi-square p-value
Not enough numbers within group cells to compute a valid chi square value
Table 3.
Demographics Based on Histologic Subtype
| Alveolar/Undifferentiated | Embryonal/botryoid | ||
|---|---|---|---|
| N eval (%) | N eval (%) | P-valuea | |
| Number of subjects | 34 | 21 | |
| Age, <20 years | 29/34 (85) | 12/21(58) | 0.056 |
| Males/Evaluable (%) | 15/34 (44) | 13/21(62) | |
| KPS ≥90 | 27/34(79) | 14/20 (67) | .291 |
| Tumor burden, > 5 cm | 21/14(66) | 12/9(75) | .834 |
| Primary site of tumor | 31 | 18 | 0.095 |
| Extremity/limb girdle | 15 (48) | 3 (16) | |
| Head and neck | 8 (26) | 8 (42) | |
| Truncal | 1 (3) | 1 (5) | |
| Visceral/retropreritoneal | 7 (23) | 5 (26) | |
| Relapse pre-transplant (N=32) | 12 (37) | 8 (38) | 0.965 |
| Response to 1st line of therapy | 30 | 19 | 0.115 |
| Complete response | 14 (53) | 5 (26) | |
| Partial response | 10 (33) | 6 (32) | |
| Stable/Progressive disease | 2 (7) | 4 (21) | |
| Not evaluable | 2 (7) | 4 (21) | |
| Disease status prior to conditioning | 33 | 21 | 0.247 |
| Complete response | 23 (70) | 10 (48) | |
| Partial response | 7 (24) | 7 (8) | |
| Stable/Progressive disease | 2 (6) | 3 (14) | |
| Time from dx to tx months (range) | 10 (3–83) | 10 (4–27) | 0.734 |
Abbreviations: CNS = central nervous system; KPS=Karnofsky score at transplant; BM=bone marrow; dx=diagnosis; tx=transplant.
Chi-square p-value
Not enough numbers within group cells to compute a valid chi square value
Outcome
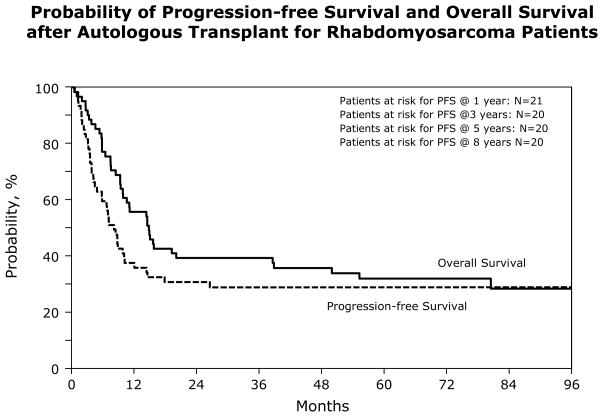
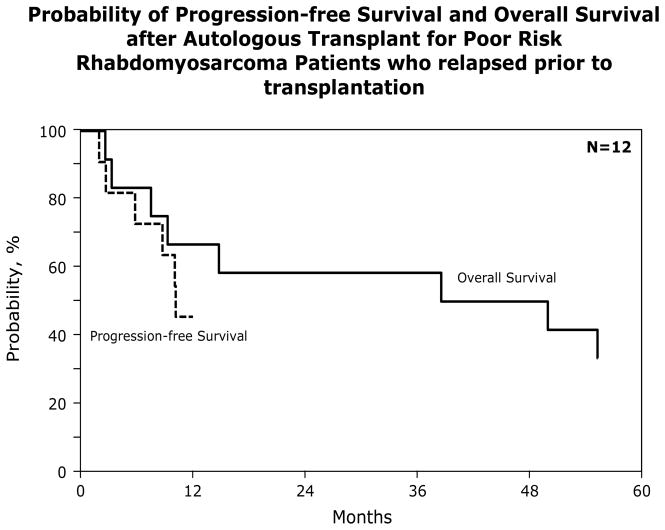
At a median follow-up of 78 months for survivors, 19/62 (31%) subjects are alive. Of those who died, 91% died of their primary malignancy. For the entire group, the TRM at 1 year was 5% (95% CI, 1–12); the 5 year PFS and OS were 29% (95% CI, 18–41) and 32% (95% CI, 21–44) respectively. Most failures occurred within the 1st year after transplant (59%) with only 2 subjects relapsing after a year and none after 3 years (Figure 1). There were no significant differences in the TRM, relapse rate or the 5 year PFS and OS when we compared outcomes between subjects with poor risk vs. good risk histology: [5 year PFS 31 (95% CI, 15–49) vs. 25% (95% CI, 9–46); p = 0.658 and OS 29 (95% CI 14–47) vs. 30% (95% CI 12–51); p = .943] (Table 5). There were also no differences in 5 year PFS [32 (95% CI 18–48) vs. 26% (95% CI 10–48); p = 0.644] or OS [36 (95% CI 22–52) vs. 29% (95% CI 12–49); p = 0.532] between those transplanted in 1st remission vs. relapse (Table 6). Twelve subjects with poor risk histology were transplanted after relapse. Their 5 year PFS was 36% (95% CI: 12 – 65) and their survival was 33% (Figure 2). This was not different from the 34% survival rate observed for those with poor risk histologies transplanted in 1st remission (p = 0.955). Likewise those with good risk histology transplanted in 1st remission had a similar outcome to those transplanted in relapse (38% vs. 25%; p=0.270).
Figure 1.
Probability of Progression Progression-free Survival and Overall Survival after Autologous Transplant for Rhabdomyosarcoma Patients
Table 5.
Univariate probabilities comparing subjects with alveolar/undifferentiated histology to subjects with embyronal/botryoid histology who received an autologous transplant for rhabdomyosarcoma between 1989 and 2003, reported to the CIBMTR.
| Alveolar/undifferentiated |
Embryonal/botryoid |
||||
|---|---|---|---|---|---|
| Outcome of interest | N (eval) | Probability (95% CI) | N(eval) | Probability (95% CI) | P-valuec |
| Treatment related mortalitya | 34 | 23 | |||
| @ 1 year | 6 (1–18)% | -- | -- | ||
| @ 3 years | 10 (2–24)% | -- | -- | ||
| @ 5 years | 10 (2–24)% | -- | -- | ||
| Progression/relapsea | 34 | 23 | |||
| @ 1 year | 48 (31–66)% | 75 (54–91)% | 0.046 | ||
| @ 3 years | 59 (41–76)% | 75 (54–91)% | 0.232 | ||
| @ 5 years | 59 (41–76)% | 75 (54–91)% | 0.232 | ||
| Progression free survivalb | 34 | 23 | |||
| @ 1 year | 45 (28–63)% | 25 (9–46)% | 0.136 | ||
| @ 3 years | 31 (15–49)% | 25 (9–46)% | 0.658 | ||
| @ 5 years | 31 (15–49)% | 25 (9–46%) | 0.658 | ||
| Overall survivalb | 34 | 23 | |||
| @ 1 year | 62 (44–79)% | 55 (33–76)% | 0.621 | ||
| @ 3 years | 45 (27–63)% | 30 (12–51) % | 0.282 | ||
| @ 5 years | 29 (14–47)% | 30 (12–51) % | 0.943 | ||
Probabilities of relapse, treatment-related mortality, platelet & neutrophil engraftment were calculated using the cumulative incidence function.
Probabilities of overall survival and progression free survival were calculated using the Kaplan-Meier product limit estimate.
Pointwise P-value
Table 6.
Univariate probabilities comparing subjects who relapsed prior to transplant to subjects who never relapsed. All subjects received an autologous transplant for rhabdomyosarcoma between 1989 and 2003, reported to the CIBMTR.
| No relapse prior to tx |
Relapsed prior to tx |
||||
|---|---|---|---|---|---|
| Outcome of interest | N (eval) | Probability (95% CI) | N(eval) | Probability (95% CI) | P-valuec |
| Treatment related mortalitya | 38 | 21 | |||
| @ 1 year | 5 (1–15)% | -- | N/A | ||
| @ 3 years | 5 (1–15)% | -- | N/A | ||
| @ 5 years | 5 (1–15)% | -- | N/A | ||
| Progression/relapsea | 38 | 21 | |||
| @ 1 year | 54 (38–70) % | 74 (52–90)% | 0.135 | ||
| @ 3 years | 63 (47–77) % | 74 (52–90)% | 0.400 | ||
| @ 5 years | 63 (47–77) % | 74 (52–90)% | 0.400 | ||
| Progression free survivalb | 38 | 21 | |||
| @ 1 year | 41 (26–57) % | 26 (10–48) % | 0.275 | ||
| @ 3 years | 32 (18–48) % | 26 (10–48) % | 0.664 | ||
| @ 5 years | 32 (18–48) % | 26 (10–48) % | 0.664 | ||
| Overall survivalb | 38 | 21 | |||
| @ 1 year | 53 (37–68) % | 62 (41–81) % | 0.487 | ||
| @ 3 years | 39 (25–55) % | 43 (23–64) % | 0.800 | ||
| @ 5 years | 36 (22–52) % | 29 (12–49) % | 0.532 | ||
Probabilities of relapse, treatment-related mortality, platelet & neutrophil engraftment were calculated using the cumulative incidence function
Probabilities of overall survival and progression free survival were calculated using the Kaplan-Meier product limit estimate.
Pointwise P-value
Figure 2.
Probability of Progression Progression-free Survival and Overall Survival after Autologous Transplant for Poor Risk Rhabdomyosarcoma Patients who relapsed prior to transplantation
DISCUSSION
Event-free survival and survival of subjects with RMS has improved significantly over the past 20 years. Supportive care and outcomes of transplants have also improved. A review by Weigel and colleagues evaluated reports containing 389 subjects transplanted before 2000. They concluded that there was no benefit to transplants in 1st remission or after relapse(13). We sought to determine whether this was still true in a more recent cohort.
Our study is one of the largest autotransplant analyses for subjects with RMS, and the only one in the past 10 years. Because of the rarity of RMS advances in standard therapy(14–18) and the prior negative reports of transplants(5–9, 13, 19, 20) this series is still small. However, data collected is quite extensive and we believe that some conclusions are possible. Most of the subjects were high-risk having either an unfavorable histology (61%) and/or metastases at diagnosis (67%) including a substantial proportion with bone marrow involvement). Only one subject had botryoid histology, the most favorable RMS histology. Approximately two-thirds were transplanted as consolidation of a 1st remission but less than one-half of this group was still in CR, with another one-third typically transplanted after a second chemotherapy-induced remission. Overall one-third of the subjects are alive and disease-free at a median of 78 months post-transplant.
Results of transplant for relapsed disease compare favorably with prior reports although subject-selection may bias this comparison. The largest transplant series to date (98 subjects) for relapsed RMS was reported by the EBMT in 1997(9). Median survival was 8 months; and only 20% of subjects survived long-term. The German-Austrian Pediatric BMT group reported 36 cases. Four of 9 subjects transplanted in relapse survived long-term(19). Most other series had <10 subjects(20). In Weigel’s review, of 51 patients transplanted in second or third CR or with active disease 3 year survival was 12%. Thus, although numbers of subjects transplanted after relapse in our series is limited and selection–biases may operate, their 35% long-term PFS is encouraging. Results of transplants are also encouraging for the few subjects with alveolar or undifferentiated histology transplanted after a relapse who had a 36% (95% CI, 12–65;) 5 year PFS. Analyzing conventional therapy after relapse, the Intergroup Rhabdomyoma Study Group in 1999 determined the 5-year survival rate after relapse for subjects with either alveolar and undifferentiated histologies was only 5%(8). They also found that the few long-term survivors in this group were those who relapsed after having had localized-disease at diagnosis that was completely excised; all others died. However, the subjects we analyzed in this subgroup were predominately transplanted in a CR after salvage chemotherapy. Thus, responsiveness to salvage chemotherapy may be a requirement for long-term survival.
Most autotransplant studies in RMS have focused on subjects receiving transplants as consolidation of high-risk disease (typically defined as metastases at presentation). This strategy of transplanting subjects with the poorest prognosis at diagnosis in 1st remission is similar to the approach that has been used effectively in treating patients with neuroblastoma and Ewing sarcoma. Carli et al. evaluated autotransplants in 52 subjects with RMS who presented with metastases (a group in which survival is approximately 25% with best standard care)(5). Requirements for entry were a CR after 6 courses of chemotherapy. Preparative regimens were predominantly melphalan- based. They compared transplant outcomes to outcomes of 44 subjects who received an additional 6 cycles of chemotherapy which they termed “contemporaneous controls”. There was no significant difference in the two groups in prognostic factors yet the 3-year event-free survival was 30% at 3 years for the transplant group, vs. 19% for those receiving chemotherapy. This difference was not significant but there was little power in the analysis because a small sample size. A statistically significant difference in time to relapse was seen however favoring transplants.
Other studies of transplants for high-risk subjects in CR1 are less well-controlled but showed similar results. Boulad and coworkers treated 26 newly diagnosed patients with RMS (21), extraosseous Ewings (2) and undifferentiated sarcoma (2) with intensive chemotherapy followed by split course radiotherapy(6). Subjects achieving a CR or PR received high-dose melphalan and etoposide. Their two-year survival was 56% (95% CI 36–76%) and PFS was 53% (95% CI 33–76%). Comparing their results to historical controls not consolidated with transplant, who had a 2-year PFS of 30%, they felt that the difference was sufficient to recommend further trials with a more abbreviated course of conventional therapy. To improve outcome, intensification of the preparative regimen has also been tested. Adding doxorubicin and vincristine to total body radiation (TBI) and high-dose cyclophosphamide Horowitz and colleagues(7) in transplanted patients with RMS in CR after induction-therapy for un-resectable alveolar or embryonal trunk and extremity RMS. All 19 subjects not in CR after induction died of disease-progression. Three of 7 subjects in CR who decided not to receive a transplant were long-term survivors, whereas 20 of 65 (31%) who received consolidation autotransplants were long-term event-free survivors. They concluded that TBI plus high-dose chemotherapy failed to improve outcomes. Our subjects transplanted in 1st remission were typically high-risk and while they had disease responsive to chemotherapy, only 40% were in CR, and only 25% had an embryonal histology. Nevertheless, these subjects had a similar outcome to the selected first CR subjects in the aforementioned studies. This could reflect improvements in induction chemotherapy over the past 10 years, or better supportive care in this more recent series, including a shift from bone marrow to blood cells grafts. Like the older studies, we believe that the favorable results in this recent series continue to support the development of randomized trials both in first remission for high risk disease and after relapse for those with disease responsive to chemotherapy.
Table 4.
Univariate probabilities of outcomes of subjects who underwent autologous transplantation for Rhabdomysarcoma, between 1989 and 2003, reported to the CIBMTR.
| Outcome of interest | N (eval) | Probability (95% CI) |
|---|---|---|
| ANC>0.5 × 109/La @ 28 days | 62 | 97 (91–100) % |
| Platelet recovery ≥ 20 × 109/La @ 28 days | 58 | 50 (37–63) % |
| Transplant-related mortalitya | 62 | |
| @ 1 year | 5 (1–12) % | |
| @ 3 years | 8 (3–17) % | |
| @ 5 years | 8 (3–17) % | |
| Progression/relapsea | 62 | |
| @ 1 year | 59 (47–71) % | |
| @ 3 years | 63 (50–75) % | |
| @ 5 years | 63 (50–75) % | |
| Progression free survivalb | 62 | |
| @ 1 year | 36 (24–48) % | |
| @ 3 years | 29 (18–41) % | |
| @ 5 years | 29 (18–41) % | |
| Overall survivalb | 62 | |
| @ 1 year | 56 (43–68) % | |
| @ 3 years | 39 (28–52) % | |
| @ 5 years | 32 (21–44) % |
Probabilities of relapse, treatment-related mortality, platelet & neutrophil engraftment were calculated using the cumulative incidence function
Probabilities of overall survival and progression free survival were calculated using the Kaplan-Meier product limit estimate.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries;; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pappo AS, Shapiro DN, Crist WM, Maurer HM. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13:2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 2.Crist WM, Garnsey L, Beltangady MS, et al. Prognosis in children with rhabdomyosarcoma: a report of the intergroup rhabdomyosarcoma studies I and II. Intergroup Rhabdomyosarcoma Committee. J Clin Oncol. 1990;8:443–452. doi: 10.1200/JCO.1990.8.3.443. [DOI] [PubMed] [Google Scholar]
- 3.Rodary C, Gehan EA, Flamant F, et al. Prognostic factors in 951 nonmetastatic rhabdomyosarcoma in children: a report from the International Rhabdomyosarcoma Workshop. Med Pediatr Oncol. 1991;19:89–95. doi: 10.1002/mpo.2950190204. [DOI] [PubMed] [Google Scholar]
- 4.Breitfeld PP, Meyer WH. Rhabdomyosarcoma: new windows of opportunity. Oncologist. 2005;10:518–527. doi: 10.1634/theoncologist.10-7-518. [DOI] [PubMed] [Google Scholar]
- 5.Carli M, Colombatti R, Oberlin O, et al. High-dose melphalan with autologous stem-cell rescue in metastatic rhabdomyosarcoma. J Clin Oncol. 1999;17:2796–2803. doi: 10.1200/JCO.1999.17.9.2796. [DOI] [PubMed] [Google Scholar]
- 6.Boulad F, Kernan NA, LaQuaglia MP, et al. High-dose induction chemoradiotherapy followed by autologous bone marrow transplantation as consolidation therapy in rhabdomyosarcoma, extraosseous Ewing’s sarcoma, and undifferentiated sarcoma. J Clin Oncol. 1998;16:1697–1706. doi: 10.1200/JCO.1998.16.5.1697. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz ME, Kinsella TJ, Wexler LH, et al. Total-body irradiation and autologous bone marrow transplant in the treatment of high-risk Ewing’s sarcoma and rhabdomyosarcoma. J Clin Oncol. 1993;11:1911–1918. doi: 10.1200/JCO.1993.11.10.1911. [DOI] [PubMed] [Google Scholar]
- 8.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- 9.Koscielniak E, Rosta G, Hartmann O, et al. High dose chemotherapy (HDC) with hematopoietic rescue (HR) in patients iwth rhabdomyosarcoma (RMS): an EBMT Solid Tumor Working party survey. Bone Marrow Transplant. 1997;19:S86. [Google Scholar]
- 10.Klein JP, Moeschberger JL. Survival Analysis: Techniques for Censored and Truncated Data. 1997:334–336. [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Weigel BJ, Breitfeld PP, Hawkins D, Crist WM, Baker KS. Role of high-dose chemotherapy with hematopoietic stem cell rescue in the treatment of metastatic or recurrent rhabdomyosarcoma. J Pediatr Hematol Oncol. 2001;23:272–276. doi: 10.1097/00043426-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 15.Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma--a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 16.Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol. 2004;22:4787–4794. doi: 10.1200/JCO.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 17.Lager JJ, Lyden ER, Anderson JR, Pappo AS, Meyer WH, Breitfeld PP. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2006;24:3415–3422. doi: 10.1200/JCO.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 18.Dantonello TM, Int-Veen C, Winkler P, et al. Initial patient characteristics can predict pattern and risk of relapse in localized rhabdomyosarcoma. J Clin Oncol. 2008;26:406–413. doi: 10.1200/JCO.2007.12.2382. [DOI] [PubMed] [Google Scholar]
- 19.Meyers PA. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr Opin Oncol. 2004;16:120–125. doi: 10.1097/00001622-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Koscielniak E, Klingebiel TH, Peters C, et al. Do patients with metastatic and recurrent rhabdomyosarcoma benefit from high-dose therapy with hematopoietic rescue? Report of the German/Austrian Pediatric Bone Marrow Transplantation Group. Bone Marrow Transplant. 1997;19:227–231. doi: 10.1038/sj.bmt.1700628. [DOI] [PubMed] [Google Scholar]