Abstract
Methylmercury (MeHg) is a ubiquitous environmental pollutant and has been shown to affect learning in vertebrates following relatively low exposures. Zebrafish were used to model long-term learning deficits after developmental MeHg exposure. Selenomethionine (SeMet) co-exposure was used to evaluate its role in neuroprotection. Embryos were exposed from 2–24 hours post fertilization to (1) MeHg without SeMet, (2) SeMet without MeHg and (3) in combination of MeHg and SeMet. In case (1), the levels of MeHg were 0.00, 0.01, 0.03, 0.06, 0.10, 0.30 µM. In case (2), the levels of SeMet were 0.00. 0.03, 0.06, 0.10, 0.30 µM. In case (3), co-exposure levels of (MeHg, SeMet) were (0.03, 0.03), (0.03, 0.06), (0.03, 0.10), (0.03, 0.30), (0.10, 0.03), (0.10, 0.06), (0.10, 0.10), (0.10, 0.30) µM. Learning functions were tested in individual adults, four months after developmental exposure using a spatial alternation paradigm with food delivery on alternating sides of the aquarium. Low levels of MeHg (<0.1 µM) exposure delayed learning in treated fish; fish exposed to higher MeHg levels were unable to learn the task; SeMet co-exposure did not prevent this deficit. These data are consistent with findings in laboratory rodents. The dorsal and lateral telencephalon are the primary brain regions in fish involved in spatial learning and memory. Adult telencephalon cell body density decreased significantly at all MeHg exposures >0.01 µM MeHg. SeMet co-exposure ameliorated but did not prevent changes in telencephalon cell body density. In summary, MeHg affected both learning and brain structure, but SeMet only partially reversed the latter.
Descriptors: developmental exposure, learning, mercury, selenium, spatial alternation, zebrafish
1. INTRODUCTION
It has been documented in a number of mammalian species that methylmercury (MeHg) exposure can induce learning deficits [1–7]. However, a lack of unequivocal results from epidemiological studies on children [8–10] has lead researchers to question the direct association of MeHg exposure to behavioral deficits and potential neurotoxicological effects. The result of this controversy has been an examination of other components in the diet that may mitigate MeHg toxicity [11, 12]. Recently, much discussion has focused on the interactions between Hg and selenium (Se), specifically selenoenzymes such as glutathione peroxidase and thyroid hormone deiodinases and their precursors [13–16]. It has been proposed that mercury (Hg), by binding to Se compounds, actually creates Se deficiencies that are the root cause of many of the toxic effects of Hg [14,16] and Se supplementation at moderate levels may provide neuroprotective effects against Hg toxicity [15, 16]. At sufficiently high concentrations, Se, however, also can induce toxic effects [17, 18].
Central to this study is the effect of Hg-Se interactions during embryo neurogenesis on adult behavior. It has been noted that developmental co-exposure to both selenomethionine (SeMet) and MeHg may result in differential effects in adult animals depending upon the specific behavior being evaluated, e.g., reduction in the severity of neurobehavioral deficits are observed only for simple reflex behaviors [19] but not for more complex learning tasks [20]. This study utilizes another vertebrate model, zebrafish, to address this discrepancy in behavioral outcomes due to developmental MeHg and SeMet co-exposure.
The fish dorsal and lateral telencephalon are critical for spatial learning of landmarks to identify foraging areas and nesting locations, and to navigate through their environments [21]. The size of these brain regions is correlated to the complexity of the individual’s spatial environment as telencephalic ablations lead to decreased spatial learning [22–26]. Within the goldfish telencephalon, specific regions relate to specific forms of learning as indicated by ablation experiments; the lateral telencephalon relates to avoidance learning, whereas the medial telencephalon appears to be responsible for reversal spatial learning [27].
Using a variety of learning paradigms, e.g., conditioned aversion, conditioned reinforcement tasks, and prey capturing abilities, behavioral impairments in both rodents and fish have been identified after exposure to environmental contaminants, including MeHg [28–32]. While the effect of MeHg on spatial learning abilities in fish has not yet been examined, developmental exposures to lead (Pb2+) or ethanol induced decreases in spatial learning abilities in zebrafish [33]. Since analyses of potential changes in telencephalon architecture or function were not recorded in that study, fundamental mechanisms of behavioral alteration could not be identified. This study utilizes zebrafish (Danio rerio) to assess some basic neurobehavioral alterations induced by developmental exposure to MeHg because of its utility as a vertebrate model system for examining the early neurogenesis of critical pathways that control specific, reproducible, easily observable behaviors [34, 35].
2. METHODS AND RESULTS
2.1 Treatment of Glassware and Plasticware
All laboratory materials made of plastic were washed thoroughly in a 10% solution of a nontoxic, biodegradable detergent (Simple Green™; Sunshine Makers, Inc., Huntington Harbour, CA), rinsed repeatedly in ultra-pure Milli-Q™ water (Millipore Corp., Medford, MA), and immersed in a 30mM Na4EDTA (Fisher Scientific, Hanover Park, IL) solution overnight to remove all surface adsorbed metal ions; glassware was washed and rinsed similarly but immersed in a 10% HNO3 (Fisher Scientific, Hanover Park, IL) solution overnight. Glass and plasticware were then rinsed in ultra-pure Milli-Q™ water.
2.2 Breeding and Egg Collection
Adult female (Tupfel long fin strain, Zebrafish International Resource Center, Eugene, OR) and male (golden leopard strain, Ekkwill Waterlife Resources, Gibsonton, FL) zebrafish were housed separately, and acclimated for several weeks prior to the initiation of experiments. Different strains were used to facilitate differentiation between sexes, as zebrafish do not show prominent signs of sexual dimorphism. Fish were maintained at 26–28°C on a 14-hour light and 10-hour dark cycle in a flow-through buffered, de-chlorinated water system at the Aquatic Animal Facility of the University of Wisconsin-Milwaukee Children’s Environmental Health Sciences Center. All experimental procedures were approved by the University of Wisconsin-Milwaukee Animal Care and Use Committee. Zebrafish were bred in 2-L plastic aquaria with a 1/8” nylon mesh false bottom to protect fertilized eggs from being consumed by the adults. Eggs were collected ≤ 2 hours post fertilization (hpf), counted, and placed into metal-free, glass culture dishes (100 mm diameter × 50 mm depth; N = 100 eggs/dish) in E2 medium [36] (each liter contains 0.875 g NaCl, 0.038 g KCl, 0.120 g MgSO4, 0.021 g KH2PO4, and 0.006 g Na2HPO4)
2.3 Exposure Regimen
Methylmercury (MeHg; >98% purity) was obtained from ICN Biomedicals (Aurora, OH). Seleno-L-methionine (SeMet; >98% purity) was obtained from Sigma Chemicals (St. Louis, MO). Collected eggs (N = 100 eggs/dish; < 2 hpf) were rinsed twice in MeHg-free E2 medium (as determined by ICP-MS analysis) and transferred to metal-free glass dish (100 mm diameter × 50 mm depth) containing 100 ml of E2 medium with either only MeHg at 0.0, 0.01, 0.03, 0.06, 0.10, 0.30 µM; or only SeMet at 0.0, 0.03, 0.06, 0.10, 0.30 µM; the 0.0 µM MeHg and 0.0 µM SeMet sets were identical. These levels of developmental MeHg exposures were found to alter adult zebrafish visual startle responses; the above levels of SeMet co-exposures were found to reduce the behavioral response only at the highest concentrations [19]. In addition, developmental co-exposures (2–24 hpf) of 0.03 or 0.1 µM MeHg and 0.03, 0.06, 0.10, or 0.30 µM SeMet were also used, i.e., 8 separate combinations (refer to Table 3A for results of all combinations). These levels were found to mitigate MeHg-induced visual startle response alterations [19]. Higher concentrations were not used as 0.6 µM MeHg or SeMet were at or above the LC50. At 24 hpf the embryos were rinsed in MeHg-free E2 medium and a subsample was analyzed for Hg and Se content by ICP-MS. The remainder were raised in MeHg-free E2 medium (28°C; 14L:10D). Fry were fed vinegar eels twice each day starting at day 5 post hatch regardless of treatment until large enough to consume Artemia nauplii. Juveniles and adults were fed Aquarian™ flake food (Aquarium Pharmaceuticals, Inc., Chalfont, PA) in the morning and Artemia nauplii in the afternoon. Based upon this and previous studies, there are no significant differences in embryo, larval, juvenile, or adult mortality or number of developmental malformations at the stated concentrations of either MeHg or SeMet. However, there are few data quantifying Se levels in these diets or if these potential sources of added Se have any effect on behavioral outcomes of MeHg-exposed fishes [37].
Table 3.
| Table 3A: Co-Exposure Data Values = ppb Hg or Se/100 embryos | ||||
|---|---|---|---|---|
| MeHg = 0.03 | MeHg = 0.10 | |||
| SeMet (µM) | Hg | Se | Hg | Se |
| 0.03 | 1.12 | 0 | 4.31 | 0 |
| 1.17 | 0 | 3.46 | 0 | |
| 1.11 | 0 | 3.95 | 0 | |
| 0.06 | 0.92 | 0.00 | 3.18 | 0 |
| 1.09 | 0.16 | 3.19 | 0 | |
| 1.09 | 0.25 | 2.54 | 0 | |
| 0.10 | 1.08 | 0.36 | 3.26 | 0 |
| 1.02 | 0.42 | 3.29 | 0 | |
| 1.03 | 0.20 | 4.00 | 0 | |
| 0.30 | 0.82 | 1.77 | 1.49 | 0.85 |
| 1.03 | 2.68 | 1.25 | 0.51 | |
| 0.87 | 1.63 | 1.14 | 0.83 | |
| Table 3B: 24 hpf Hg and Se average for each treatment combination Values = mean and SEM of 3 replicates | ||||
|---|---|---|---|---|
| 0.030 µM MeHg | 0.100 µM MeHg | |||
| Hg | Se | Hg | Se | |
| SeMet =0.030 | 1.13 (0.02) |
0.00 | 3.91 (0.25) |
0.00 |
| SeMet =0.060 | 1.03 (0.06) |
0.14 | 2.97 (0.22) |
0.00 |
| SeMet =0.100 | 1.04 (0.02) |
0.33 | 3.52 (0.24) |
0.00 |
| SeMet =0.300 | 0.91 (0.06) |
2.03 | 1.29 (0.10) |
0.73 |
2.4 Embryo Hg and Se analysis
Metal analyses follow previously published protocols [19]. For each exposure concentration of MeHg and SeMet and co-exposure regimen of MeHg and SeMet the data were collected in 3 replicates. For each replicate, 100 eggs were collected after 24 hpf, rinsed twice in Hg-free E2 medium, placed in Teflon™ microvials (7.0 ml) with the chorion intact, and acid digested (2.0 ml of: 80 ml ICP-MS grade, ultrapure HNO3 + 0.5 ml ICP-MS grade, ultrapure gold (Au) diluted to 1.0 l with Milli-Q™ water) in a microwave oven (MARS 5, CEM Corp., Matthews, NC). Gold was added to the digestion solution of both control and treated eggs to scavenge any Hg that might otherwise adsorb to the vessel wall and be unavailable for analysis. The “closed vessel” digestion was carried out under a temperature controlled program (25°C to 130°C at 5°C per minute, held at 130°C for 10 min, cooled to room temperature). Samples were decanted into 20 ml autosampler vials and brought to a final volume of 10.0 ml with the addition of 9.0 ml digestion solution. Mercury and selenium were measured with a MicroMass Platform inductively coupled plasma-mass spectrophotometer (ICP-MS) (Manchester, UK) equipped with a CETAC ASX 500 autosampler (Waters Corp, Medford, MA) under MassLynx NT software control for element measurements. Appropriate calibration standards were prepared from a 10µg mL−1 (in 5% ICP-MS grade HNO3) Hg or Se standard (CertiPrep, Metuchen, NJ). A calibration curve was constructed by ICP-MS analysis of 1–100 ppb Hg.and Se. The solvent system solution blank was 5% HNO3, 0.1% HCl and 500 ppb Au in ICP-MS grade, ultrapure water (18 MegOhm). Acids used were double-distilled ICP-MS grade (Optima, Fisher Scientific). All analyses were measured in the SIR Mode (Single Ion Recording) for 60 s. Separate analyses (N = 3/exposure) were conducted on the exposure media to verify concentrations. Data were recorded as medium MeHg or SeMet concentration vs. tissue residue (ppb) as the level of these substances in the body represents the effective exposure regimen for the developing embryo. Thus, control embryos may have residual levels of either mercury or selenium. The collected data are given in Table 1A, Table 2A, and Table 3A. Since the specific speciation of either mercury or selenium was not determined, they are simply identified both in the tables and in the general text as Hg or Se without regard to oxidation state.
Table 1.
| Table 1A: MeHg Exposure Only Data: Values = ppb Hg/100 embryos | |||||
|---|---|---|---|---|---|
| MeHg (µM) | |||||
| 0 | 0.01 | 0.03 | 0.06 | 0.10 | 0.30 |
| 0.87 | 1.12 | 2.73 | 4.29 | 9.41 | 16.74 |
| 0.79 | 1.48 | 3.05 | 5.17 | 7.27 | 13.04 |
| 0.72 | 1.46 | 2.65 | 5.55 | 9.75 | 21.39 |
| Table 1B: One-way ANOVA: Embryo Hg versus MeHg Exposure | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | F | P |
| MeHg | 5 | 570.03 | 114.01 | 34.51 | 0.000 |
| Error | 12 | 39.65 | 3.30 | ||
| Total | 17 | 609.68 | |||
| Table 1C: CI for treatment mean minus control mean: Control = 0.00 µM MeHg | |||
|---|---|---|---|
| MeHg (μM) | Lower | Center | Upper |
| 0.01 | −3.75 | 0.56 | 4.87 |
| 0.03 | −2.29 | 2.02 | 6.32 |
| 0.06 | −0.10 | 4.21 | 8.52 |
| 0.10 | 3.71 | 8.02 | 12.32 |
| 0.30 | 11.96 | 16.26 | 20.57* |
Dunnett's comparisons with a control. Family error rate = 0.05. Individual error rate = 0.0133. Critical value = 2.90. Control = level (0) of MeHg.
P < 0.001.
Table 2.
| Table 2A: SeMet Exposure Only Data Values = ppb Se/100 embryos | |||||
|---|---|---|---|---|---|
| SeMet (µM) | |||||
| 0.00 | 0.03 | 0.06 | 0.10 | 0.30 | |
| 0.18 | 1.07 | 1.28 | 1.40 | 3.24 | |
| 0.00 | 0.51 | 0.69 | 0.28 | 2.62 | |
| 0.00 | 0.34 | 0.53 | 0.31 | 2.94 | |
| Table 2B: One-way ANOVA: Embryo Se versus SeMet Exposure | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | F | P |
| SeMet | 4 | 14.67 | 3.67 | 22.46 | 0.000 |
| Error | 10 | 1.63 | 0.16 | ||
| Total | 14 | 16.30 | |||
| Table 2C: CI for treatment mean minus control mean: Control = 0.00 µM SeMet | |||
|---|---|---|---|
| SeMet (µM) | Lower | Center | Upper |
| 0.03 | −0.37 | 0.58 | 1.53 |
| 0.06 | −0.18 | 0.77 | 1.73 |
| 0.10 | −0.35 | 0.60 | 1.56 |
| 0.30 | 1.92 | 2.87 | 3.83* |
Dunnett's comparisons with a control. Family error rate = 0.05. Individual error rate = 0.0161. Critical value = 2.89. Control = level (0) of SeMet.
P < 0.001.
2.4.1. Statistical Analysis
Uptake values of SeMet alone or incombination with MeHg have been previously reported [19] and verified in this study.
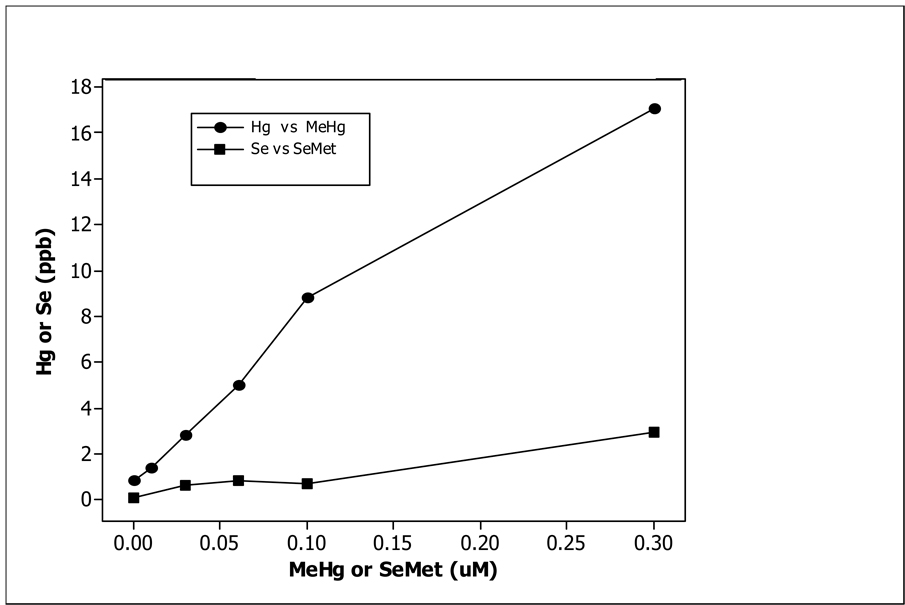
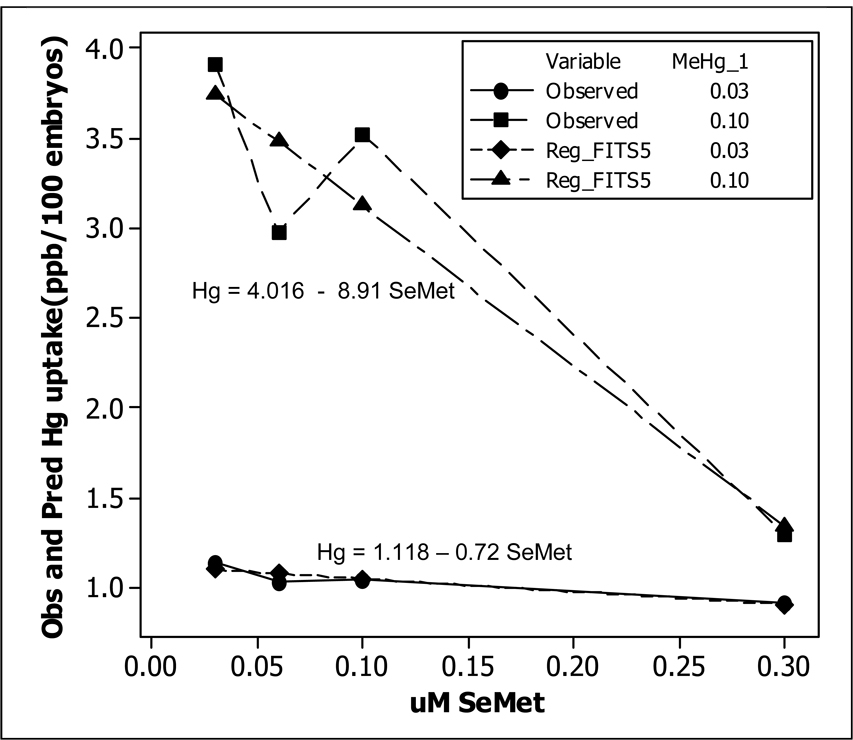
Data from MeHg exposure only are given in Table 1A. Developmental waterborne exposure from 2–24 hpf resulted in a dose-dependent uptake of MeHg as measured by total Hg (ANOVA, p<0.05). The data for SeMet exposure only are given in Table 2A. Total Se uptake remained low for treatments below 0.3 µM. The data from the co-exposure of MeHg and SeMet are given Table 3A. Co-exposure dramatically influenced the uptake of both MeHg and SeMet at exposure combinations of 0.1 µM MeHg over a range of SeMet concentrations but not at 0.03 µM MeHg over the same range of SeMet concentrations. Mean Hg uptake and mean Se uptake from MeHg exposure data in Table 1A and SeMet exposure data in Table 2A are plotted in Figure 1. It clearly shows that Hg was transported into the embryo at a much higher rate than Se. The co-exposure data in Table 3A was analyzed using a Two-Way ANOVA procedure (Table 3B). The interaction term between MeHg and SeMet is highly significant with P value of 0. Fig. 2 shows the plot of the mean Hg uptake under 8 different co-exposure levels and predicted values of MeHg uptake with a co-exposure of either 0.03 or 0.10 µM SeMet. At 0.03 µM MeHg, Hg uptake did not change much as the level of SeMet is increased to 0.30 µM. The t-test comparing these two means has a P value of 0.3063. This was expected since MeHg level was low to begin with. However, for 0.10 µM MeHg, the Hg uptake decreased sharply when the SeMet level is increased to 0.30 µM. The t-statistic comparing these two means has a P value of 0. The ANOVA table also indicates that the main effects of SeMet were highly significant (Table 4B). The main effects of MeHg were also significant (Table 4A).
Figure 1.
Concentration (ppb) of Hg (•---•) or Se (■---■) in newly fertilized zebrafish embryos (24 hpf) at each concentration (µM) of MeHg or SeMet.
Figure 2.
Observed concentration (ppb) of Hg in newly fertilized zebrafish embryos (24 hpf) after co-exposure to either 0.03 (•---•) or 0.10 (■---■) µM MeHg and either 0.03, 0.05, 0.10, or 0.30 µM SeMet. Predicted concentration (ppb) of Hg in newly fertilized zebrafish embryos (24 hpf) after co-exposure to either 0.03 (♦---♦) or 0.10 (▲---▲) µM MeHg and either 0.03, 0.05, 0.10, or 0.30 µM SeMet. Linear regression model for each concentration of MeHg vs. SeMet displayed.
Table 4.
| Table 4A: Two-way ANOVA: Embryo Hg versus MeHg, SeMet Exposure | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | F | P |
| MeHg | 1 | 21.49 | 21.49 | 311.48 | 0.000 |
| SeMet | 3 | 6.94 | 2.31 | 33.52 | 0.000 |
| Interaction | 3 | 5.07 | 1.69 | 24.52 | 0.000 |
| Error | 16 | 1.10 | 0.07 | ||
| Total | 23 | 34.61 | |||
| Table 4B: Two-way ANOVA: Embryo Se versus MeHg, SeMet Exposure | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | F | P |
| MeHg | 1 | 1.16 | 1.16 | 23.80 | 0.000 |
| SeMet | 3 | 7.70 | 2.57 | 52.59 | 0.000 |
| Interaction | 3 | 1.55 | 0.52 | 10.58 | 0.000 |
| Error | 16 | 0.78 | 0.049 | ||
| Total | 23 | 11.19 | |||
Since the levels of MeHg and SeMet are quantitative variables, a regression model was developed to model Hg uptake as a function of MeHg and SeMet. This allowed the prediction of Hg uptake for any level of MeHg and SeMet within a reasonable range. The regression equation is: Hg = − 0.124 + 41.4 MeHg + 2.79 SeMet − 117 MeHg*SeMet. The adjusted R-square is 91.9% indicating a good fit. The P value for the significance of the interaction term is 0. At 0.03 µM MeHg the equation above reduces to: Hg = 1.118 – 0.72 SeMet. At 0.1 µM MeHg, the equation reduces to: Hg = 4.016 – 8.91 SeMet. These two lines are plotted in Fig. 2. Collectively, the ANOVA analysis and regression model indicate that a) Hg uptake increased significantly when MeHg was increased even in the presence of high levels of SeMet, b) increasing SeMet decreased the Hg uptake significantly as SeMet level was increased, and c) SeMet has a greater impact on whole embryo Hg levels at higher MeHg concentrations.
2.5 Spatial Alternation Task
Fish (4 months of age) developmentally exposed to various concentrations of MeHg with or without SeMet were used in the spatial alternation task. The alternation testing apparatus was based on a previously published design [38] that has been augmentted with computer automation to eliminate any cues or biases that may be introduced by the presence of the experimenter. Each 10 l aquarium (30.5 cm length × 15.3 cm width × 20.3 cm height), filled with 28°C, water (purified through reverse osmosis) containing 60 mg sea salt/L, was divided into two equal sections with a white divider that provided sufficient room at the bottom of the tank for the fish to swim from side to side. To make the left side of the tank distinguishable from the right a red- or white-colored card was attached to each wall at the end of the aquarium. Zebrafish see and react to the color red [39], therefore they would be able to distinguish between the two compartments by color. No food was delivered for at least 24 hours prior to testing allowing for the fish to be hungry and driven to perform for food. For each treatment combination 10 fish were randomly selected with each fish individually placed into 10 aquaria (1/aquarium). Adult zebrafish developmentally exposed to MeHg with or without SeMet were randomly assigned a number using a random number generator that identified which test tanks each individual fish occupied. The order in which each individual fish was tested was determined randomly based upon a random number generator. During the experiment, the researcher was blind to the treatment to avoid biases. The fish were tested 8 at a time, one/test aquarium, until 28 trials were completed. The total time required for each test was, therefore, 9 hours. The testing was computer automated with eight animals being tested per day. A total of 3 weeks was required to complete testing of all experimental subjects.
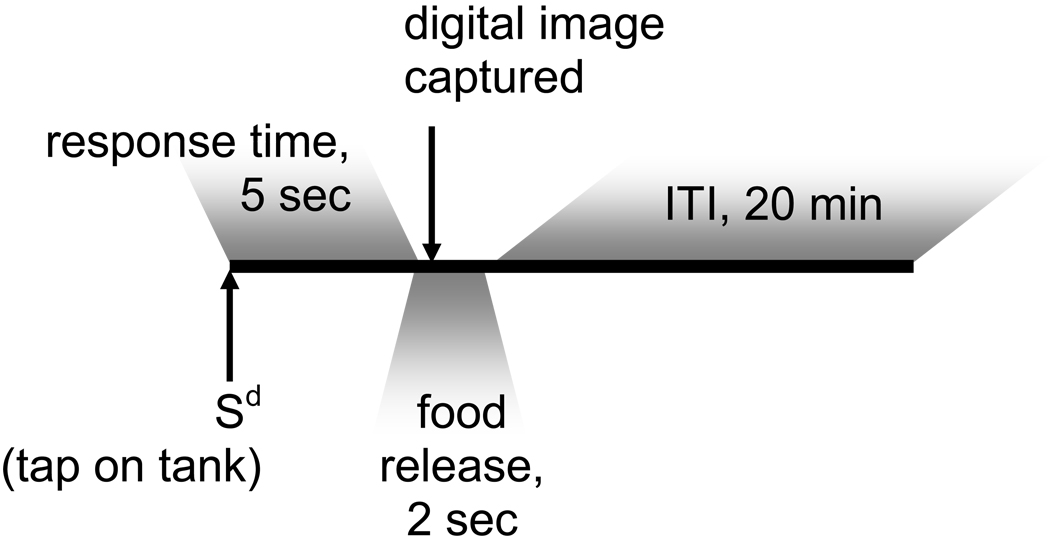
The procedure for testing learning, an operant/respondent technique, utilized an external stimulus to autoshape a behavioral response (Fig. 3). Animals started at or near random chance or 50% correct choices made. This methodology was published previously and adapted from previous mammalian models [33,38]. Briefly, each trial started with (1) a tap on the aquarium followed by (2) a sort delay of 5 second, followed by (3) food release in one side of the aquarium, followed by (4) behavior observation. If the fish found the food then it was recoded as a success. Otherwise it was recorded as a failure. At the end each trial, percentage of successes out of 10 was calculated. This was recorded as observed value of P. After each trial a gap of 20 minutes was provided for the fish to prevent satiation. After 20 minutes gap, the next trial started. Specifically, trials began with a conditional discriminative stimulus (Sd), a light tap at the center of each aquarium (1 fish/aquarium; 8 aquaria/session; 10 fish/exposure regimen; 8 concurrent analyses with individual fish from each exposure regimen randomly chosen; age at testing = 4 months) by a mechanical apparatus. This provided a cue for the animal to make a decision. It was not possible to place and maintain a similar orientation within the aquarium for all fish nor was it necessary since the experimental design called for response to an acoustical rather than a visual stimulus. After a short 5 sec delay, food was released into one side of the tank by a tube connected through a peristaltic pump to a beaker of a diluted concentration of Artemia, and a digital image of all aquaria was captured within 100 msec of the initiation of food dispersal. The trial then ended after food release and picture capture, and the 20 minute intertrial interval began the next trial. To avoid satiation and to maintain motivation, only approximately 5 Artemia were dispensed per trial. If the fish was not on the correct side at time of food release, the food fell through a grate to the bottom of the tank and was missed. In order to be optimally successful, the fish must learn to move to the correct side upon sensing the Sd. Previous experience indicated that without a food drop regardless of response, the fish would not learn. Food presentation on the next trial (after the 20 min intertrial interval) was on the opposite side of the tank and this pattern of food delivery alternation occurred for a total of 28 trials. A response was considered correct if the fish was present on the side of food release at the time of the digital image capture after food dispersal. These data were recorded and the correct responses were pooled by treatment for statistical analysis. Learning was defined to have occurred under a certain dosing scenario if the estimated maximum probability of a correct response was 75% or higher and the estimated time to achieve the half-way point to this maximum was within the experimental time frame of 28 trials [38] (see Supplemental Table for raw data).
Figure 3.
Sequence of events in a trial of the spatial alternation task. Food release alternates tank side on successive trials (e.g., the left side was fed on all odd numbered trials and the right side was fed on all even numbered trials). Digital images were captured 100 ms after the initiation of food release. Sd = Discriminative stimulus; ITI = Intertrial interval.
2.5.1. Statistical Analysis
All animals were assumed to have started at or near 50% correct responses, i.e., random chance. The probability of correct response under a given dosing scenario was modeled as described previously [33, 38]. The equation is repeated here for clarity:
The observed percentages of successes are given in the Supplemental Table for each treatment combination. These observed percentages of successes were used to estimate the two learning curve parameters, A and T, from the sigmoidal learning curve. These parameters are: “ A” which represents the amount of total learning, with an ultimate probability “P” of correct response equal to 0.5 + A, and “T” which represents the time, as measured in number of trials, until half of that total learning is achieved. For example, in Table 5 the value of A for 0.0 µM MeHg is 0.31. Therefore, the total amount of learning is 0.31 above the initial threshold or 0.5 + 0.31 = 0.81, which is above the criterion of 0.75. The estimated learning curve was used to decide whether the learning goal was achieved or not. The learning criterion was set at P ≥ 0.75, i.e., learning did not take place if A ≤ 0.25 and T > 28. If the learning goal was met at trial t0, i.e. P(t0) ≥ 0.75, then t0 was taken as the learning score. The SAS nonlinear modeling procedure NLIN was employed for estimation, with 95% confidence intervals used for testing the statistical significance of parameter estimates. These learning score results are recorded in Table 5 and Table 6 for all treatment combinations.
Table 5.
Summary Statistics for Adult Learning Curve Estimates based on data in Supplemental Table.
| Group (µM) | A | 95% CI for A | T | 95% CI for T | Significant? | Trial # Criterion Reacheda |
|
|---|---|---|---|---|---|---|---|
| MeHg | |||||||
| .00 | .31* | (.23,.39) | 9.1* | (5.7,12.6) | yes | 12 | |
| .01 | .10* | (.05,.16) | 2.5 | (−2.3,7.3) | no | ---- | |
| .03 | ---- | ---- | 40.5* | (14.9,66.2) | no | ---- | |
| .06 | .01 | (−.10,.12) | 5.8 | (−130.8,142.4) | no | ---- | |
| .10 | ---- | ---- | 36.7* | (21.6,51.7) | no | ---- | |
| .30 | ---- | ---- | 38.0* | (19.7,56.3) | no | ---- | |
| SeMet | |||||||
| .00 | .30* | (.20,.40) | 6.3* | (2.3,10.3) | yes | 12 | |
| .01 | .15* | (.09,.22) | 1.7 | (−1.5,4.9) | no | ---- | |
| .03 | .40* | (.02,.78) | 21.2* | (10.8,31.5) | yes# | 25 | |
| .06 | .17* | (.11,.23) | 7.8 | (3.1,12.6) | no | ---- | |
| .10 | .18* | (.08,.29) | 8.7* | (1.1,16.3) | yes | ---- | |
| .30 | .12 | (.06,.18) | 2.6 | (−1.8,6.9) | no | ---- | |
| MeHg+SeMet | |||||||
| .03 | .03 | .15 | (−.04,.34) | 5.8 | (−.4,32.1) | no | ---- |
| .03 | .06 | .08 | (−.02,.19) | 9.2 | (.7.9,26.3) | no | ---- |
| .03 | .10 | .15 | (−.03,.33) | 13.3 | (−2.5,19.2) | no | ---- |
| .03 | .30 | .20* | (.06,.33) | 12.6* | (3.0,22.2) | yes | ---- |
| .10 | .03 | .05 | (−.16,.27) | 13.4 | (−43.3,70.1) | no | ---- |
| .10 | .06 | .16 | (−.42,.74) | 21.9 | (−17.6,61.4) | no | ---- |
| .10 | .10 | .13 | (−.22,.48) | 19.4 | (−12.4,51.3) | no | ---- |
| .10 | .30 | .10* | (.05,.16) | none | ---- | no | ---- |
A = total amount of learning
T = # trials to achieve half of total learning
a parameter estimate is statistically significant
mean trial # criterion reached estimated by mathematical estimation of curve (see text)
criterion reached but a trial number outside the range of control 95% confidence interval for T.
Table 6.
| Table 6A: Mean ± S.E. of % Cell Volume | ||||||
|---|---|---|---|---|---|---|
| MeHg (µM) |
0 | 0.01 | 0.03 | 0.06 | 0.10 | 0.30 |
| Mean | 77.09 | 63.30 | 58.26 | 62.37 | 45.45 | 48.52 |
| Std. Error | 5.02 | 5.60 | 4.66 | 4.36 | 7.23 | 9.12 |
| n | 8 | 8 | 8 | 8 | 8 | 8 |
| Table 6B: One-way ANOVA: Cell Volume vs MeHg (µM) | ||||||
|---|---|---|---|---|---|---|
| Source | df | SS | MS | F | P | |
| MeHg | 5 | 4623.80 | 924.80 | 27.11 | 0.000 | |
| Error | 38 | 1296.30 | 34.10 | |||
| Total | 43 | 5920.00 | ||||
| Table 6C: CI for treatment mean minus control mean: Control = 0.00 µM MeHg | ||||||
|---|---|---|---|---|---|---|
| MeHg (µM) | Lower | Center | Upper | |||
| 0.01 | −21.48 | −13.78 | −6.09 | |||
| 0.03 | −26.52 | −18.82 | −11.13 | |||
| 0.06 | −22.42 | −14.72 | −7.02 | |||
| 0.10 | −39.34 | −31.64 | −23.95 | |||
| 0.30 | −36.99 | −27.57 | −18.14 | |||
Dunnett#x00027;s comparisons with a control. Family error rate = 0.05. Individual error rate = 0.0121. Critical value = 2.64. Control = level (0) of MeHg
In the exposure regimen consisting of MeHg alone, the learning criterion was met only with 0.0 µM MeHg. At all other exposure levels of MeHg criterion was not achieved, indicating that even a small amount of MeHg was detrimental to learning. In the exposure regimen consisting of SeMet alone, the learning criterion was met only at 0 and 0.03 µM SeMet. At all other levels of SeMet criterion was not achieved, indicating that higher levels of SeMet were also toxic.
Hg and Se uptake were associated with decreased learning success (Table 5). In the co-exposure regimen of MeHg and SeMet, the learning never took place in any treatment combination. This suggests that the co-exposure of SeMet along with MeHg was not able to mitigate Hg-induced learning deficits. Since learning did not take place at higher levels of SeMet even in the absence of MeHg, it was not clear in the co-exposure experiment what caused the learning deficit, higher level of SeMet or the presence of Hg.
Table 5 displays the parameter estimates, their statistical significance, and the status of each MeHg/SeMet combination scenario in terms of meeting the learning criteria and the mean trial number at which criterion for learning was reached for each combination of MeHg and SeMet used in the study. Observed and fitted data are detailed in the Supplemental Table. To declare the learning criteria to have been met, it is required that the data met the previously stated definition plus the parameters are both statistically significant (different from zero). If either parameter is not statistically distinguishable from zero, this serves as an indication that the data do not clearly demonstrate any particular pattern, let alone learning. In Table 5 this requirement for dual significance is shown where a given co-exposure has an asterisk for both the parameter A and T. It turns out that the learning criteria were met in only three MeHg/SeMet combination scenarios and that the criteria were only met in cases where both parameter estimates turned out to be statistically significant (Table 5). The three scenarios were both controls for the MeHg and the SeMet study and for the study using 0.03 µM SeMet with no MeHg. In the MeHg study, there were three concentrations for which the timing parameter, T, was statistically significant but well beyond the study’s upper limit of 28 trials (Table 5). Thus, any inference for these situations would be based on severe extrapolation. This is reflected by the non-convergence that resulted for the amount parameter, A, in these three cases and is reflected in Table 5 in which most exposure combinations do not reach criterion. In the MeHg/SeMet study, there two other scenarios, that of 0.03 µM MeHg with 0.30 µM SeMet, and 0.10 µM SeMet with no MeHg where both parameter estimates were statistically significant (Table 5). In these cases, however, the amount parameter A was below the criterion level of 0.25. Raw data and modeled sigmoidal curve for each of these four scenarios with both parameters statistically significant are given in Supplemental Table. The raw data consists of the relative frequencies of correct responses at each trial number.
An examination of Table 5 leads to the following observations. The vehicle-treated control fish (0.0 µM MeHg + 0.0 µM SeMet) reached criterion, i.e., correctly predicted the side of food delivery at least 75% of the time, <10 trials as estimated. By the end of the session (28th trial) the controls responded correctly more than 80% of the time. However, even the lowest treatment of MeHg, 0.01 µM, induced a delaying effect on learning in that criterion was not reached by trial 28. For several MeHg treatments (0.03, 0.1, and 0.3 uM), there appears to be evidence of late learning, although even at trial 28 the responses were not significantly different from random choice. Fish treated with SeMet below 0.3 uM reached criterion by the 28th trial. Fish co-exposed to MeHg and SeMet showed signs of gradual learning, although criterion was not reached within 28 trials and the response was not significantly different from random choice; the effect of co-exposure was greater when the embryos were exposed to 0.30 µM MeHg over a range of SeMet concentrations than 0.01 µM MeHg over the same range of SeMet concentrations.
2.6 Brain Histopathology
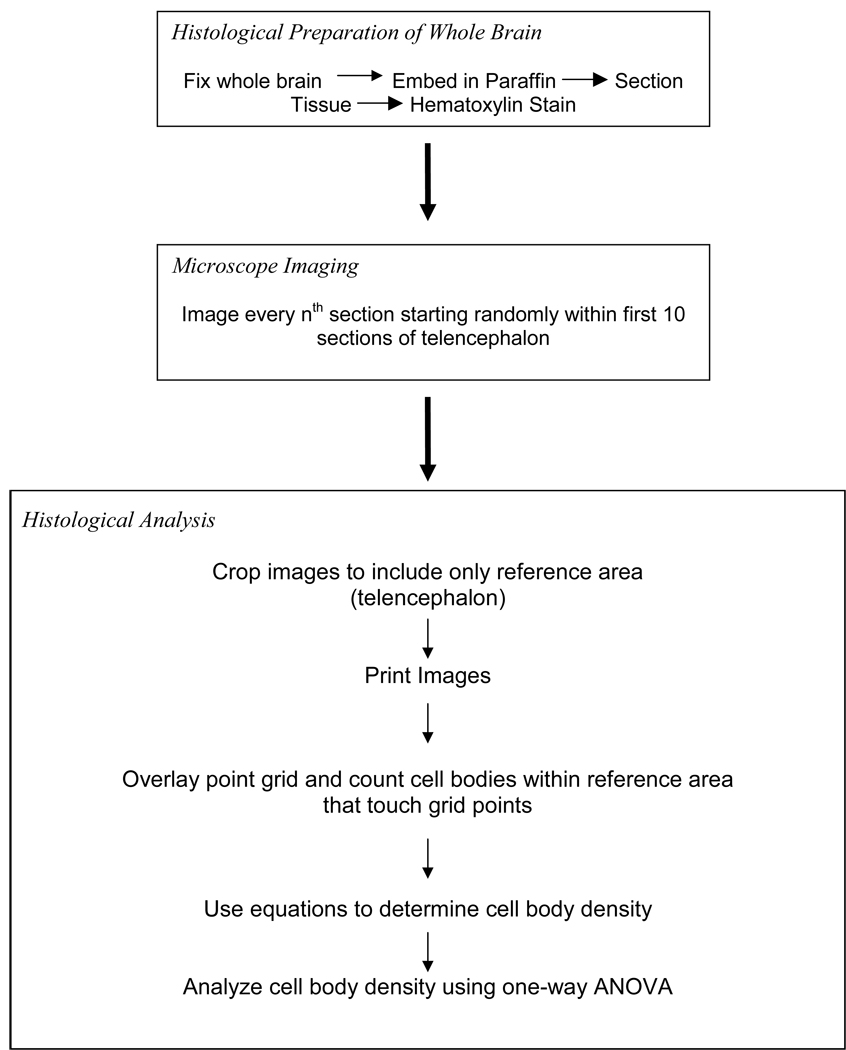
The procedure for preparing and analyzing the histological sections is outlined in Fig. 4. Non-tested fish (4 months of age; N = 8) of each treatment regimen were euthanized using an overdose of MS-222 (tricaine methanesulfonate; Argent Chemical Laboratories; Redmond, WA), an anesthetic commonly used for aquatic vertebrates, and decapitated. Heads were fixed in 4% paraformaldehyde (5 ml/fish) overnight, rinsed, decalcified overnight in Cal-Ex (Fisher Scientific), and dehydrated in a graded series of ethanol (30%, 50%, 70%, and 100%) for 2 hrs each. Samples were cleared with xylene and embedded with paraffin in a vacuum oven (56°C; 15 psi). Slices (20µm thickness cut using a rotary microtome) were placed on microscope slides coated with a Paramarker™ (Electron Microscopy Sciences, Hatfield, PA) adhesive to dry overnight on a heated slide drier. Tissues were stained with hematoxylin to highlight cell bodies for counting.
Figure 4.
Flow chart for methodology of histological section preparation and analysis.
Every nth section was analyzed to prevent an over sampling bias (recounting a cell because it appears in multiple sections). The “n” was determined by the total number of sections in the reference area divided by 10. A random number generator determined the start point between the first and tenth section and digital images were acquired from the histological samples between the start point and the caudal end of reference space. The reference space was determined rostrally by the presence of brain tissue on top of the olfactory bulb and caudally by the appearance of the optic tectum. Images were acquired with a digital camera (Olympus DP70) mounted to a light microscope (Olympus BX60) at 20× magnification. The images were printed, and a transparency point grid was placed over the reference space to facilitate quantization. Both the total number of points on the reference space and the number of cell bodies that touched a point were counted to determine the reference and cell body volumes. Within the histology slide section, three of the reference area edges were determined by the medial end of one hemisphere, and the dorsal and lateral end of the brain tissue. The ventral edge was created by a line extended from the ventral edge of the lateral telencephalon to the ventral edge of the medial telencephalon. The microscope was randomly focused within the section layer, so all tissue had an equally random chance of being analyzed. Only those cell bodies that were in focus were counted within the reference space. All brain regions were identified according to Wullimann et al. [40].
The total cell body density by area fraction or % cell volume, i.e., Dcell = Vobj/Vref (vs. cell body volume which was not calculated) was calculated by first using a point grid to count the number of points within the reference area and the number of points that were touched by a cell body. These data points used to find the reference volume (Vref) and total object volume (Vobj) through the equations [41]:
where:
ΣA = sum of the reference area on each section (µm2) = ΣPref * area per point
t = average section thickness (µm)
k = the sampling interval, (e.g. every 5th section)
ΣPobj = total number of points touching a cell body on each section
ΣPref = total number of points within the reference space on each section
2.6.1. Statistical Analysis
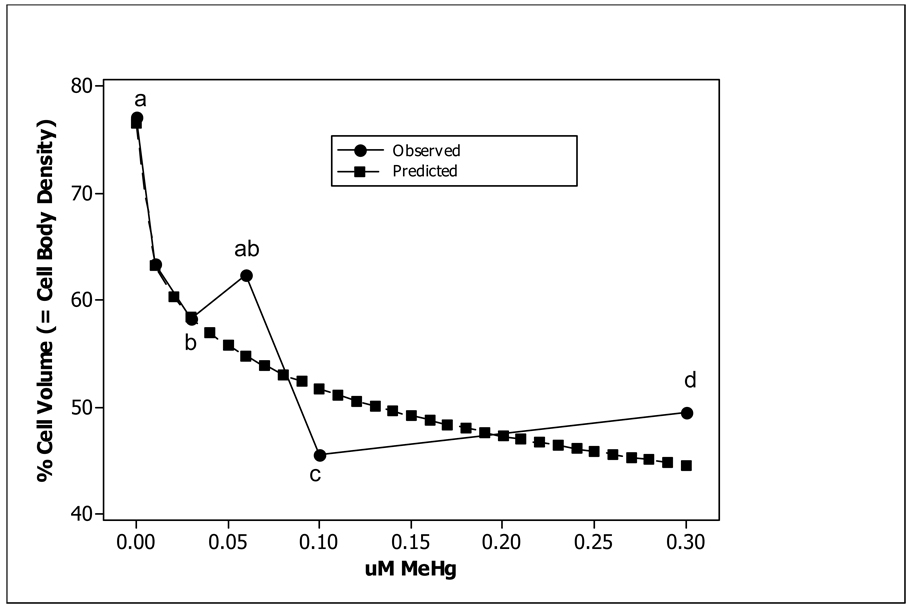
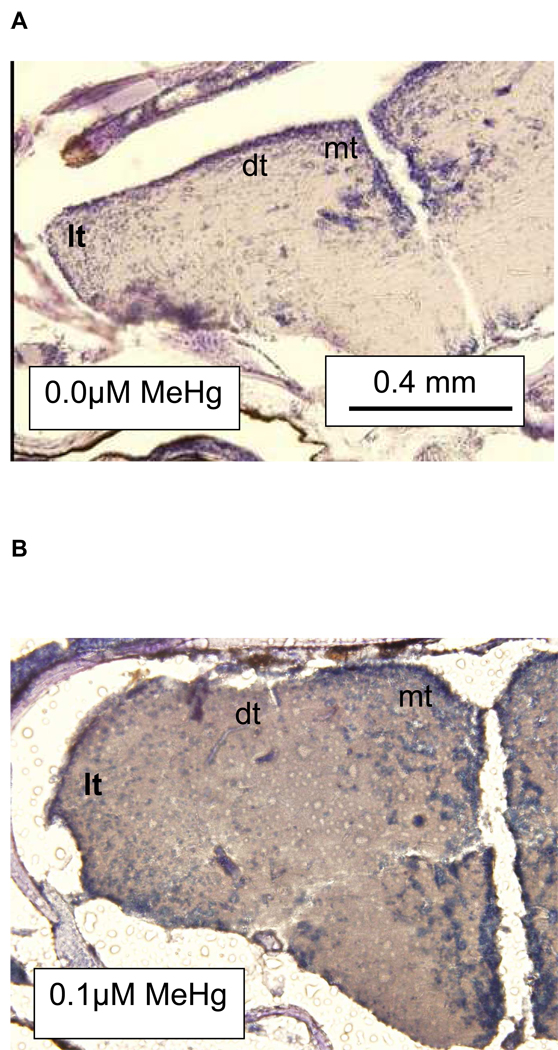
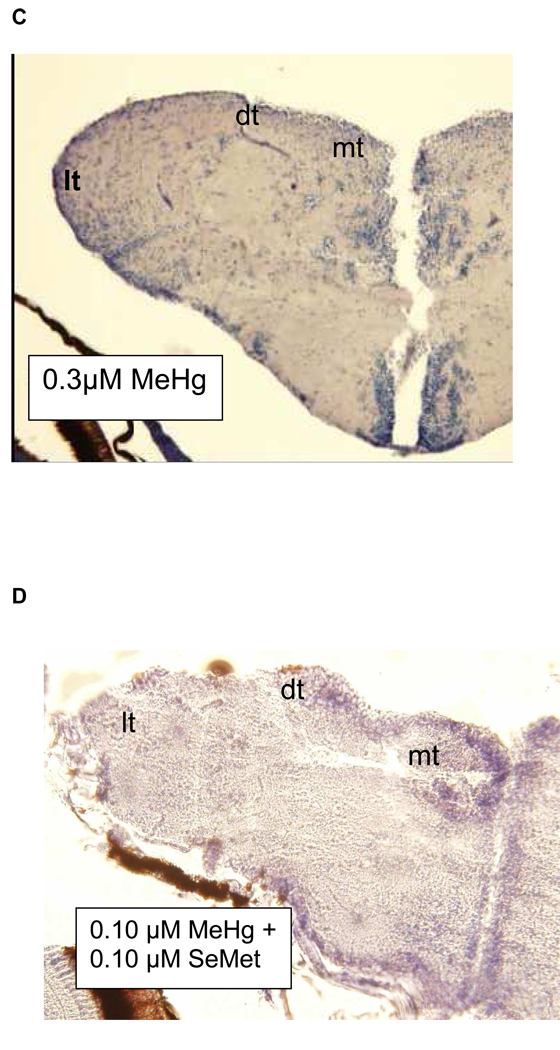
Histopathological analyses of brain tissue showed a trend of decreased percent cell body density in the telencephalon with increased exposure concentration (Fig. 5; Tables 6A–C). To verify the model (Fig. 5), future experiments will need to asses changes in cell body density due to developmental exposures between 0.1 and 0.3 µM MeHg. Through a simple visual examination of histological samples parallel reductions in the dorsal, lateral, and medial telencephalon were observed (Figs. 6A–C). The indeterminate results observed in the learning tests were mirrored by the histological analysis of the telencephalon from zebrafish exposed as embryos to a combination of MeHg and SeMet. A visual examination of Fig, 6D suggests that in those fish co-exposed to 0.1 µM MeHg + 0.1 µM SeMet the lateral telencephalon had a highly reduced cell body density, the dorsal telencephalon some reduction, and the medial telencephalon little, if any, reduction of cell bodies. The overall cell body density in all areas of the telencephalon while not as low as that observed from adult zebrafish exposed developmentally to either 0.1 or 0.3 µM MeHg alone was not as high as that observed from adult zebrafish exposed developmentally to 0–0.06 µM MeHg alone (Fig. 5; Tables 6A–C).
Figure 5.
Cell body density as percent cell volume in telencephalon (dorsal, lateral, and medial) in adult zebrafish (N = 8) exposed as embryos to various concentrations of MeHg (●) or 0.10 µM MeHg + 0.10 µM SeMet (○). The regression line (r2=0.85) is drawn for the MeHg exposure only. The letters represent levels of significance (ANOVA, P<0.05), where non-overlapping letters denote significant differences.
Figure 6.
Histological sections of zebrafish telencephalon illustrating changes in neuron cell body density (blue staining). Fish were developmentally exposed to A. 0.0 µM MeHg + 0.0 µM SeMet (control), B. 0.1 µM MeHg, C. 0.3 µM MeHg, or D. 0.10 µM MeHg + 0.10 µM SeMet from 2–24 hpf. lt = lateral, dt = dorsal, and mt = medial telencephalon. Cell body volume for telencephalon based upon counts of all sections as described in text.
4. DISCUSSION
All exposure regimes used in this study were sublethal, were similar to those used with other fish species, represent environmentally relevant concentrations, and produced similar LC50s for embryo exposures [42–47]. Although several studies have indicated greater MeHg uptake in fish than observed in this study [47–50], none of those involved developmental exposures targeted at and isolated to the earliest stages of embryonic neuron formation (the first 24 hpf in zebrafish). It is expected, therefore, that the values of both total Hg and Se uptake as reported here (Fig. 1) and in Weber et al. [19] are significantly less than those reported elsewhere, even if the exposure concentrations were of a similar order of magnitude. Additionally, values reported in this study were for whole egg, i.e., embryo chorion, and perivitelline fluid, and likely underestimates the tissue concentrations in the embryo. This, however, further emphasizes that the early stages of zebrafish embryo development are exceedingly sensitive to even small amounts of MeHg, at least as it pertains to adult learning responses and the neuronal development within key regions of the brain associated with spatial associations. We have observed a lack of significant developmental abnormalities in embryos exposed to even the higher concentrations of MeHg [44]. Therefore, the behavioral changes reported in this study likely are due to fundamental changes in neural organization and function rather than changes in anatomical structures.
Ontogenetic critical periods in fishes have been described as a function of determining post-hatch behavioral patterns [51]; it is likely that in zebrafish the first 24 hpf are such a time frame as it relates to sensitivity to toxic chemicals during early neuro- and organogenesis. This time frame is comparable to the first trimester of human development in which Hg-sensitive selenoenzymes, such as thyroid hormone deiodinases and their precursors [13–16], are required for proper neuronal development. Selenium plays a critical role in the development of both humans and fishes [52, 53]. Other studies have demonstrated, as well, that fish embryos are sensitive to environmental contaminants and that these effects can be observed in larval, juvenile, and adult behavioral expressions [54–60].
This study provided support for long-term structural changes (specifically a decrease in cell body density) in dorsal, lateral, and medial telencephalon (Figs. 6A–C) as a potential mechanism of adult learning deficits after developmental MeHg exposure. Since the uptake of MeHg is concentration-dependent (Tables 1A–C), the behavioral and structural alterations observed in this study can be traced back to the amount of MeHg the embryo accumulated (Fig. 1, Fig. 2). It is noteworthy that the MeHg accumulated during the first day of embryogenesis was depurated to levels too low to be detected through ICP-MS methods by the initial larval stages [57]. The adult effects observed in this study, therefore, were not due to any detectable residual body burdens of MeHg but likely the result of early, permanent alterations in the structure and function of the zebrafish brain. This possibility is suggested by both in vivo and in vitro studies on Hg-induced alterations in neural development and growth [61–64].
Interestingly, SeMet co-exposure affects MeHg concentrations in the embryo, at least at high SeMet and high MeHg exposures (Fig. 2; Tables 3A, B). Further investigations are required to determine whether the effect of Se co-exposure on whole embryo Hg levels is due to alterations in uptake, metabolism, or excretion. Uptake of Hg parallels increased uptake of Se in both embryos and fetuses [65, 66]. Data from Weber et al. [19] suggest that, while interactions also may occur at lower SeMet:MeHg ratios, it may not be sufficient to reduce behavioral alterations if both are present in the exposure media at higher concentrations. The lack of evidence in this study that SeMet reduced MeHg-induced learning deficits (Table 5, Table 6) parallels results observed in mammalian models [20]. It also is possible that the absence of improvements in learning abilities after SeMet co-exposure reflects potential limitations of this experimental paradigm as a model both for the effects of ecological trophic transfers of Hg or Se and for human environmental health. Currently, the distribution of Se in its various inorganic and organic forms within the embryo or adult zebrafish is not understood, although it is likely that SeMet is the form found in fish tissue [67] and is the most bioavailable form of Se [68]. This, in turn, relates to our current lack of information regarding the transfer of SeMet within specific food chain scenarios. Given these model limitations, this study represents an important step in understanding the potential neurobehavioral outcomes in fishes, and by extension humans, co-exposed to multiple environmental chemicals.
The underlying theme of spatial alternation tasks is to assess the rate at which fish are capable of learning predictable changes in spatial patterns over time [69]. In this study, predictive abilities by each individual fish were aided by an environmental cue (a tap on the aquarium wall; Sd). This allowed the fish to recognize that a trial had begun, remember which side of the aquarium food was last presented, and predict which side food was now to be provided. Especially important for these studies is the extent to which that rate is altered due to exposure to environmental contaminants. Two types of learning appear to be occurring, both spatial learning and, to a lesser extent, identification of the conditional stimulus itself. This provides a working memory framework. Spatial learning in fishes is accomplished primarily in the dorsal and lateral telencephalon and organizes functions similar to that accomplished by the frontal cortex of the human brain [26, 66, 70–73]. Changes in learning ability may, therefore, be reflected in changes in the structure and/or function of these brain regions. Since these experiments used developmental exposures, the potential role of MeHg in life-long learning deficits was evaluated.
In our preliminary studies, we observed that those fish that had learned the task would eat the presented food, immediately swim to the other side of the tank and remain there for the duration of the 20 min intertrial interval. Once the next tap came for the next trial, the fish was already on the correct side of the tank, waiting for the food. Additionally, this was set up as a positive reinforcement trial. Therefore, we assume that the fish is acting as it does in order to get food. If the fish was not on the appropriate side of the tank at food release (and also at the time the photograph was taken), the fish would not have time to get the reward before the food fell into through nylon mesh at the bottom of the tank. Therefore, if the fish did display any stereotypic behavior, it would have to be over by the time of food release (and the photo) or it would miss its opportunity for reinforcement. This behavior was apparently learned rather quickly by those fish not developmentally exposed to MeHg (Table 5).
Low concentrations of developmental MeHg caused delayed learning, while high concentrations caused extreme impairment or even inhibition of learning (Table 5). The intermediate MeHg-treated fish showed evidence of learning, although they did not reach criterion (Table 5). Similar observations were noted in fathead minnows (Pimephales promelas) exposed to inorganic mercury as adults [47]. Therefore, a dose-response effect appears to exist between MeHg uptake (Fig. 1) and learning abilities (Table 5). Since the fish exposed only to SeMet showed slight, albeit statistically insignificant, reductions in learning (Table 5), it was expected that SeMet would have little or no affect on improvements in learning abilities in MeHg-exposed fish (Table 5), as it appeared to do for the simpler escape response [19].
The histopathological analysis examined one mechanism that might explain the delayed learning observed during the spatial alternation task. The downward trend of telencephalon cell body density with increased exposure levels at or above 0.01 µM MeHg supported the hypothesis that developmental exposure to MeHg caused life-long morphological changes in the region of the zebrafish brain associated with adult spatial learning (Fig. 5, Fig. 6; Tables 6A–C).
Developing zebrafish brains exposed to MeHg showed alterations similar to humans developmentally exposed to MeHg, e.g., neuronal loss in brain structures analogous to the cerebral cortex and cerebellum [74, 75]. Since deficits were seen in the behavioral task and brain morphology in all regions of the telencephalon was altered (Figs. 6A–C; Tables 6A–C), perhaps abnormal growth within the telencephalon, especially the dorsal and lateral regions, contributed to the delay in spatial task learning. These conclusions do not rule out other mechanisms of the central or peripheral nervous systems, e.g., alterations in sensory neuron function [19, 57, 76–79], that could contribute to decreases in sensitivity to the mechanical, odor, or visual stimuli required for learning the spatial alternation task and/or information processing that would result in the appearance of a learning deficit. Reduced sensory neuron activation may lessen the fish’s ability to extract information about its environment and facilitating an appropriate decision-making process.
It is interesting to note that when 0.10 µM MeHg is co-exposed with 0.10 µM SeMet during development, that the cell body density in the adult zebrafish telencephalon resembles that observed after developmental exposure to 0.001–0.06 µM MeHg (Fig. 5). This parallels findings in Medaka (Oryzias latipes) in which a 1 Hg: 1Se molar ratio was required to reduce liver abnormalities induced by Hg [80]. Although the addition of SeMet to the embryo medium that included MeHg resulted in a decrease of brain morphological alterations, the cell body density, particularly in the lateral and dorsal telencelphalon, still resembled a MeHg-only exposure level at which learning is delayed (Table 5, Table 6; Fig. 5, Fig. 6). Importantly, that particular co-exposure regime did not yield an improvement in learning abilities (Table 5).
In conclusion, it is clear that embryonic MeHg exposure does induce long-term effects on brain development, especially in those regions critical for coordinating learning, even at the lowest concentrations used in this study. However, co-exposures to the Hg antagonist Se in the form of SeMet did not reduce those effects either in terms of regional brain cell body numbers or in behavioral outcomes. Because this is in contrast to previous findings that such SeMet co-exposures ameliorated reduced visual startle responses in response to developmental MeHg exposures [19], questions still remain as to the mechanisms involved in these observations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Heather Owen (UW-Milwaukee Department of Biological Sciences Electron Microscopy Facility) and Henry Tomasiewicz (UW-Milwaukee Children’s Environmental Health Sciences Center Imaging and Histology Facility Core) for assisting with preparing the histology slides and Frank Laib (University of Wisconsin-Milwaukee Department of Chemistry Instrumentation Laboratory) for assistance with chemical analyses. The authors also thank the anonymous reviewers who provided invaluable suggestions to improve this manuscript. Research was supported by NIEHS grants ES04184 and ES012891, and the Great Lakes Native American Research Center for Health (NARCH; Indian Health Service 1U26 94 00014). Research was conducted under University of Wisconsin-Milwaukee IACUC protocol 05–06 #17.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110 suppl 1:11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newland MC, Reile PA, Lanagston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ Health. 2003;2:8–18. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newland MC, Warfvinge K, Berlin M. Behavioral consequences of in utero exposure to mercury vapors: alterations in lever-press durations and learning in squirrel monkeys. Toxicol Appl Pharmacol. 1996;139:374–386. doi: 10.1006/taap.1996.0178. [DOI] [PubMed] [Google Scholar]
- 5.Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposures to lead or methylmercury: reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicol Appl Pharmacol. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert SG, Burbacher TM, Rice DC. Effects of in utero methylmercury exposure on a spatial delay alternation task in monkeys. Toxicol Appl Pharmacol. 1993;123:130–136. doi: 10.1006/taap.1993.1229. [DOI] [PubMed] [Google Scholar]
- 7.Cagiano R, De Salvia MA, Renna G, Tortella E, Braghiroli D, Parenti C, et al. Evidence that exposure to methylmercury during gestation induces behavioral and neurochemical changes in offspring of rats. Neurotoxicol Teratol. 1990;12:23–28. doi: 10.1016/0892-0362(90)90108-o. [DOI] [PubMed] [Google Scholar]
- 8.Davidson PW, Myers GJ, Weiss B, Shamlaye CF, Cox C. Prenatal methyl mercury exposure from fish consumption and child development: a review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicol. 2006;27:1106–1109. doi: 10.1016/j.neuro.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Dolbec J, Mergler D, Sousa Passos CJ, Sousa de Morais S, Lebel J. Methylmercury exposure affects motor performance of a riverine population of the Tapajos river, Brazilian Amazon. Int Arch Occup Environ Health. 2000;73:195–203. doi: 10.1007/s004200050027. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger JA. Exposure assessment and initial intervention regarding fish consumption of tribal members of the Upper Great Lakes Region in the United States. Environ Res. 2004;95:325–340. doi: 10.1016/j.envres.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Passos CJ, Mergler D, Gaspar E, Morais S, Lucotte M, Larribe F, Davidson R, de Grosbois S. Eating tropical fruit reduces mercury exposure from fish consumption in the Brazilian Amazon. Environ Res. 2003;93:123–130. doi: 10.1016/s0013-9351(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 13.Cuvin-Aralar ML, Furness RW. Mercury and selenium interaction: a review. Ecotoxicol Environ Saf. 1991;21:348–364. doi: 10.1016/0147-6513(91)90074-y. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe C. Selenium deficiency and brain functions: the significance for methylmercury toxicity. Nippon Eiseigaku Zasshi. 2001;55:581–589. doi: 10.1265/jjh.55.581. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Zhao J, Li B, Liu S, Zhang P, Chai Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ Health Perspect. 2006;114:297–301. doi: 10.1289/ehp.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999;80:208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SJ, Holley KM, Buhl KJ, Bullard FA. Selenium impacts on razorback sucker, Colorado: Colorado River III. Larvae. Ecotoxicol Environ Saf. 2005;61:168–189. doi: 10.1016/j.ecoenv.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SJ. Review of selenium toxicity in the aquatic food chain. Sci Total Environ. 2004;326:1–31. doi: 10.1016/j.scitotenv.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Weber DN, Connaughton VP, Dellinger JA, Klemer D, Udvadia A, Carvan MJ., III Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol Behav. 2008;93:250–260. doi: 10.1016/j.physbeh.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicol. 2006;27:721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodson JJ. The nature and role of learning in the orientation and migratory behavior of fishes. Environ Biol Fish. 1988;23:161–183. [Google Scholar]
- 22.Pollen AA, Dobberfuhl AP, Scace J, Iglulu MM, Renn SC, Shumway CA, Hofmann HA. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav Evol. 2007;70:21–39. doi: 10.1159/000101067. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann HA. Functional genomics of neural and behavioral plasticity. J Neurobiol. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- 24.Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- 25.Odling-Smee L, Braithwaite VA. The role of learning in fish orientation. Fish Fisheries. 2003;4:235–246. [Google Scholar]
- 26.Salas C, Rodriguez F, Vargas JP, Durán E, Torres B. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci. 1996;110:965–980. doi: 10.1037//0735-7044.110.5.965. [DOI] [PubMed] [Google Scholar]
- 27.Portavella M, Vargas JP. Emotional and spatial learning in goldfish is dependent on different telencephalic pallial systems. Eur J Neurosci. 2005;21:2800–2806. doi: 10.1111/j.1460-9568.2005.04114.x. [DOI] [PubMed] [Google Scholar]
- 28.Atchison GJ, Henry MG, Sandheinrich MB. Effects of metals on fish behavior: a review. Environ Biol Fish. 1987;18:11–25. [Google Scholar]
- 29.Weir PA, Hine CH. Effects of various metals on behavior of conditioned goldfish. Arch Environ Health. 1970;20:45–51. doi: 10.1080/00039896.1970.10665540. [DOI] [PubMed] [Google Scholar]
- 30.Hartman AM. Mercury feeding schedules: effects on accumulation, retention, and behavior in trout. Trans Am Fish Soc. 1978;107:369–365. [Google Scholar]
- 31.Salzinger K, Fairhurst SP, Freimark SJ, Wolkoff FD. Behavior of the goldfish as an early warning system for the presence of pollutants in water. J Environ Sys. 1973;3:27–40. [Google Scholar]
- 32.Zhou T, Scali R, Weis JS. Effects of methylmercury on ontogeny of prey capture ability and growth in three populations of larval Fundulus heteroclitus. Arch Environ Contamin Toxicol. 2001;41:47–54. doi: 10.1007/s002440010219. [DOI] [PubMed] [Google Scholar]
- 33.Carvan MJ, III, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Ghysen A, Dambly-Chaudière C, Raible D. Making sense of zebrafish development in the Minervois. Neural Develop. 2007;2:15–28. doi: 10.1186/1749-8104-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 1993;2:269–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 36.Nüsslein-Volhard C. Zebrafish: A Practical Approach. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 37.Sorensen EM. Metal Poisoning in Fish. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 38.Williams FE, White DC, Messer WS., Jr A simple spatial alternation task for assessing memory function in zebrafish. Behav Process. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 39.Brockerhoff S, Hurley J, Niemi G, Dowling J. A new form of inherited red-blindness identified in zebrafish. J. Neurosci. 1997;17:4236–4242. doi: 10.1523/JNEUROSCI.17-11-04236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain. Berlin, Germany: Birkhäuser Verlag; 1996. [Google Scholar]
- 41.Moutin PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore, MD: Johns Hopkins University Press; 2002. Typical stereology designs; pp. 177–185. [Google Scholar]
- 42.Heisinger JF, Green W. Mercuric chloride uptake by eggs of the ricefish and resulting teratogenic effects. Bull Environ Contam Toxicol. 1975;14:665–673. doi: 10.1007/BF01685240. [DOI] [PubMed] [Google Scholar]
- 43.Sakaizumi M. Effect of inorganic salts on mercury-compound toxicity to the embryos of the Medaka, Oryzias latipes. J Fac Sci. 1980;14:369–384. [Google Scholar]
- 44.Carvan MJ, III, Weber DN. Unpublished data. [Google Scholar]
- 45.Ram A, Rokade MA, Borole DV, Zingde MD. Mercury in sediments of Ulhas estuary. Mar Pollut Bull. 2003;46:846–857. doi: 10.1016/S0025-326X(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Gupta AK. Acute toxicity of mercury to the fingerlings of Indian major carps (catla, rohu, and mrigal) in relation to water hardness and temperature. J Environ Biol. 2006;27:89–92. [PubMed] [Google Scholar]
- 47.Grippo MA, Heath AG. The effect of mercury on the feeding behavior of fathead minnows (Pimephales promelas) Ecotoxicol Environ Saf. 2003;55:187–198. doi: 10.1016/s0147-6513(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 48.Liao CY, Zhou OF, Fu JJ, Shi JB, Yuan CG, Jiang GB. Interaction of methylmercury and selenium on the bioaccumulation and histopathology in medaka (Oryzias latipes) Environ Toxicol. 2007;22:69–77. doi: 10.1002/tox.20236. [DOI] [PubMed] [Google Scholar]
- 49.Heisinger JF, Green W. Mercuric chloride uptake by eggs of the ricefish and resulting teratogenic effects. Bull Environ Contam Toxicol. 1975;14:665–673. doi: 10.1007/BF01685240. [DOI] [PubMed] [Google Scholar]
- 50.Ribeyre F, Amiard-Triquet C, Boudou A, Amiard JC. Experimental study of interactions between five trace elements—Cu, Ag, Se, Zn, and Hg—toward their bioaccumulation by fish (Brachydanio rerio) from the direct route. Ecotoxicol Environ Saf. 1995;32:1–11. doi: 10.1006/eesa.1995.1078. [DOI] [PubMed] [Google Scholar]
- 51.Browman HI. Embryology, ethology and ecology of ontogenetic critical periods in fish. Brain Behav Evol. 1989;34:5–12. doi: 10.1159/000116486. [DOI] [PubMed] [Google Scholar]
- 52.Jeong DW, Kim EH, Kim TS, Chung YW, Kim H, Kim IY. Different distributions of selenoprotein W and thioredoxin during postnatal brain development and embryogenesis. Mol Cells. 2004;17:156–159. [PubMed] [Google Scholar]
- 53.de Rosemond SC, Liber K, Rosaasen A. Relationship between embryo selenium concentration and early life stage development in white sucker (Catostomus commersoni) from a northern Canadian lake. Bull Environ Contam Toxicol. 2005;74:1134–1142. doi: 10.1007/s00128-005-0699-7. [DOI] [PubMed] [Google Scholar]
- 54.Gelert G, Heinrichsdorff J. Effect of age on the susceptibility of zebrafish eggs to industrial wastewater. Wat Res. 2001;15:3754–3757. doi: 10.1016/s0043-1354(01)00084-7. [DOI] [PubMed] [Google Scholar]
- 55.Dave G, Xiu RQ. Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch Environ Contam Toxicol. 1991;21:126–134. doi: 10.1007/BF01055567. [DOI] [PubMed] [Google Scholar]
- 56.lvarez MC, Murphy CA, Rose KA, McCarthy ID, Fuiman LA. Maternal body burdens of methylmercury impair survival skills of offspring in Atlantic croaker (Micropogonias undulates) Aquat Toxicol. 2006;80:329–337. doi: 10.1016/j.aquatox.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Weber DN. Dose-dependent effects of developmental mercury exposure on C-start escape responses of larval zebrafish Danio rerio. J Fish Biol. 2006;69:75–94. [Google Scholar]
- 58.Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P. Effects of contaminants on behavior: biochemical mechanisms and ecological consequences. BioSci. 2001;51:209–217. [Google Scholar]
- 59.Samson JC, Goodridge R, Olobatuyi F, Weis JS. Delayed effects of embryonic exposure of zebrafish (Danio rerio) to methylmercury (MeHg) Aquat Toxicol. 2001;51:369–376. doi: 10.1016/s0166-445x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 60.Weis P, Weis JS. Effects of embryonic pre-exposure to methylmercury and Hg2+ on larval tolerance in Fundulus heteroclitus. Bull Environ Contam Toxicol. 1983;31:530–534. doi: 10.1007/BF01605470. [DOI] [PubMed] [Google Scholar]
- 61.Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- 62.Parran DK, Barone S, Mundy WR. Methylmercury decreases NGF-induced TrkA autophosphorylation and neurite outgrowth in PC12 cells. Brain Res Dev Brain Res. 2003;141:71–81. doi: 10.1016/s0165-3806(02)00644-2. [DOI] [PubMed] [Google Scholar]
- 63.Leong CC, Syed NI, Lorscheider FL. Retrograde degeneration of neurite membrane structural integrity of nerve growth cones following in vitro exposure to mercury. Neuroreport. 2001;12:733–737. doi: 10.1097/00001756-200103260-00024. [DOI] [PubMed] [Google Scholar]
- 64.Miura K, Himeno S, Koide N, Imura N. Effects of methylmercury and inorganic mercury on the growth of nerve fibers in cultured chick dorsal root ganglia. Tohoku J Exp Med. 2000;192:195–210. doi: 10.1620/tjem.192.195. [DOI] [PubMed] [Google Scholar]
- 65.Prati M, Gornati R, Boracchi P, Biganzoli E, Fortaner S, Pietra R, Sabbioni E, Bernardini G. A comparative study of the toxicity of mercury dichloride and methylmercury, assayed by the Frog Embryo Teratogenesis Assay—Xenopus (FETAX) Altern Lab Anim. 2002;30:23–32. doi: 10.1177/026119290203000104. [DOI] [PubMed] [Google Scholar]
- 66.Fredriksson A, Gårdlund AT, Bergman K, Oskarsson A, Ohlin B, Danielsson B, Archer T. Effects of maternal dietary supplementation with selenite on the postnatal development of rat offspring exposed to methylmercury in utero. Pharmacol Toxicol. 1993;72:377–382. doi: 10.1111/j.1600-0773.1993.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 67.George GN, Singh SP, Prince RC, Pickering IJ. Chemical forms of mercury and selenium in fish following digestion with simulated gastric fluid. Chem Res Toxicol. 2008;21:2106–2110. doi: 10.1021/tx800176g. [DOI] [PubMed] [Google Scholar]
- 68.Deagen JT, Butler JA, Beilstein MA, Whanger PD. Effects of dietary selenite, selenocystine and selenomethionine on selenocysteine lyase and glutathione peroxidase activities and selenium levels in rat tissues. J Nutr. 1987;117:91–98. doi: 10.1093/jn/117.1.91. [DOI] [PubMed] [Google Scholar]
- 69.Braithwaite VA. Cognitive ability in fish. In: Sloman KA, Wilson RW, Balshine S, editors. Behaviour and Physiology of Fish. Amsterdam, Netherlands: Elsevier, Inc; 2006. pp. 1–37. [Google Scholar]
- 70.Lopez JC, Bingman VP, Rodriguez F, Gomez Y, Salas C. Dissociation of place and cue learning by telencephalic ablation in goldfish. Behav Neurosci. 2000;114:687–699. doi: 10.1037//0735-7044.114.4.687. [DOI] [PubMed] [Google Scholar]
- 71.Overmier JB, Hollis KL. In: Fish in the think tank: learning, memory, and integrated behavior. Kesner RP, Olton DS, editors. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1990. pp. 205–236. [Google Scholar]
- 72.López JC, Broglio C, Rodriguez F, Thinus-Blanc C, Salas C. Reversal learning deficit in a spatial but not in a cued one after telencephalic ablation in goldfish. Behav Brain Res. 2000;109:91–98. doi: 10.1016/s0166-4328(99)00167-9. [DOI] [PubMed] [Google Scholar]
- 73.Salas C, Broglio C, Duran E, Gomez A, Ocana FM, Jimenez-Moya F, Rodriguez F. Neuropsychology of learning and memory in teleost fish. Zebrafish. 2006;3:141–155. doi: 10.1089/zeb.2006.3.157. [DOI] [PubMed] [Google Scholar]
- 74.Peters MM, Hill KE, Burk RF, Weeber EJ. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol Neurodegener. 2006;19:1–12. doi: 10.1186/1750-1326-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castoldi AF, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Rev Environ Health. 2003;18:19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro CA, Fernandes LM, Carvalho CS, Cardoso RI, Turcatti NM. Acute effects of mercuric chloride on the olfactory epithelium of Trichomycterus brasiliensis. Ecotoxicol Environ Saf. 1995;31:104–109. doi: 10.1006/eesa.1995.1049. [DOI] [PubMed] [Google Scholar]
- 77.Baatrup E, Døving KB, Winberg S. Differential effects of mercurial compounds on the electroolfactogram (EOG) of salmon (Salmo salar L.) Ecotoxicol Environ Saf. 1990;20:269–276. doi: 10.1016/0147-6513(90)90006-q. [DOI] [PubMed] [Google Scholar]
- 78.Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Processes. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Dellinger J, Malek L, Beattie M. Mercury contamination of fish in the Ojibwa diet: II. Sensory evoked responses in rats fed walleye. Wat Air Soil Pollut. 1995;80:77–83. [Google Scholar]
- 80.Liao C-Y, Zhou Q-F, Fu J-J, Shi J-B, Yuan C-G. Interaction of methylmercury and selenium on bioaccumulation and histopathology in Medaka (Oryzias latipes) Environ Toxicol. 2007;22:69–77. doi: 10.1002/tox.20236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.