Abstract
To construct a sensory neuropathy model, excess pyridoxine (150 mg/kg s.i.d.) was injected subcutaneously in dogs over a period of 7 days. During the administrations period, the dogs experienced body weight reduction and proprioceptive loss involving the hindquarters. After pyridoxine administration was completed, electrophysiological recordings showed that the M wave remained at a normal state, but the H-reflex of the treated dogs disappeared at 7 days. The dorsal funiculus of L4 was disrupted irregularly in the axons and myelin with vacuolation. The dorsal root ganglia of L4, and sciatic and tibial nerves showed degenerative changes and vacuolation. However, the lateral and ventral funiculi of L4 showed a normal histopathologic pattern. Although this subcutaneous administration method did not cause systemic toxicity and effectively induced sensory neuropathy, this study confirmed the possibility of producing a pyridoxine-induced sensory neuropathy model in dogs with short-term administration.
Keywords: dog, H-reflex, pyridoxine, sensory neuropathy
Introduction
Pyridoxine, a form of vitamin B6, is an essential dietary constituent [5]. Vitamin B6 occurs in three natural forms: pyridoxol (pyridoxine), pyridoxal, and pyridoxamine. Pyridoxine is most commonly used as a dietary supplement and therapeutic agent. High dose pyridoxine is used in conditions such as premenstrual and carpal tunnel syndromes, and has been prescribed as a treatment for ingestion of the false morel mushroom, Gyromitra esculenta [4]. The rationale behind the latter treatment is that the active toxin monomethylhydrazine competitively inhibits a pyridoxine-dependent step in the synthesis of the neurotransmitter gamma-aminobutyric acid [10]. While pyridoxine deficiency causes distal, predominantly sensory neuropathy, pyridoxine has also been identified as a neurotoxicant. During the 1980s, the medical community was alerted to a neurologic disease occurring in individuals consuming large quantities of vitamin B6 for prolonged periods of time [10]. Investigators described severe sensory neuropathy of insidious onset and course associated with chronic abuse of oral pyridoxine supplements. The recommended daily oral dose is 2-4 mg in human adults. However, with daily oral doses of up to 6 g for 12-40 months, one may contract progressive sensory neuropathy manifested by sensory ataxia, diminished distal limb proprioception, paresthesia, and hyperesthesia [3,11].
There are many animal model studies based on these data. In rat studies, three intraperitoneal dosing regimens are generally employed: short term/high dose, 1,200 mg/kg/day for 1-15 days; intermediate dose, 600 mg/kg/day for 1-15 days; and long term/low dose, 100-300 mg/kg/day for up to 12 weeks [13]. Such experimental studies have confirmed the morphologic pattern of peripheral nervous system lesions in pyridoxine neurotoxicity, reflecting primary injury to the cytons of neurons residing in peripheral sensory (dorsal root, trigeminal) ganglia, with secondary degeneration of axons of affected cells [10].
There have been some experiments wherein sensory neuropathy was induced in dogs with pyridoxine overdose. However, these previous experiments used extreme doses of pyridoxine or were performed over a long period of time [5,8,9].
The incidence of neurodegenerative disorders has been increasing in the canine population, and this development requires effective countermeasures. However, canine models of specific neurodegenerative disorders are prerequisite to developing new treatments. The purpose of this study was to develop a dog model of sensory neuropathy by administering subcutaneous (SC) injections of pyridoxine over a short period of time. This differs from other experiments, in which extreme doses of pyridoxine were delivered by oral administration or were delivered over a long period of time.
Materials and Methods
Animals
Ten dogs (five beagles, three Shih tzus, one Pekingese, and one mongrel) were used for determining normal electrophysiological values. There were five male and five female dogs, all of which were around two years of age. Body weight ranged from 4 to 6 kg.
Eight dogs (four Shih-tzus, two mongrels, one Pekingese, and one Yorkshire terrier) were used for the pyridoxine-induced neuropathy study. There were four male and four female dogs, all of which were around two years of age. Body weight ranged from 4 to 6 kg. Two dogs were in the control group, and six dogs were in the experimental group.
All dogs were clinically judged to be in good health and to be neurologically normal. All experimental dogs had their own admission number issued by the Institute of Laboratory Animal Resources, Seoul National University (Korea). During the experiment, all dogs were cared for according to Animal Care and Use Guidelines from the Institute of Laboratory Animal Resources, Seoul National University. Body weight and condition were measured every morning in the test dogs.
Pharmacological treatment
Pyridoxine (Sigma, France) was diluted in a 0.9% sterile aqueous solution of sodium chloride and administered to each dog SC, once a day, in the morning, for 7 days. The pyridoxine solution was prepared immediately before each injection. Animals in the control group received a vehicle (iso-osmotic sterile aqueous solution of sodium chloride), while animals in the experimental group were administered 150 mg/kg pyridoxine SC, in a concentration of 100 mg/ml. SC injection was chosen as the route of pyridoxine administration because of convenience. The SC dosage (150 mg/kg, s.i.d. for 7 days) was decided based on the orally administered dosage of pyridoxine previously shown to induce peripheral neuropathy [5].
Postural reaction assessments
Postural reaction (wheelbarrowing, hopping, extensor postural thrust, placing, tonic neck reaction, and proprioceptive positioning) assessments were done on all dogs every morning during the test period.
Electrophysiological recordings
All dogs were pre-anesthetized with atropine (0.1mg/kg of body weight, IM). Anesthesia was induced with diazepam and was maintained with isoflurane through a semi-closed system. Subcutaneous temperature was maintained at 37-38℃. Neuropack2 (Nihon Koden, Japan) was used for all recordings. All measurements were performed in the left hindlimb of each dog.
Direct-evoked muscle potentials (DEMP, M wave) were recorded for the tibial nerve using a 1 Hz, 0.5 ms, supramaximal stimulus. Stimulating electrodes were positioned in the distal tibial nerve. A recording electrode was positioned in the plantar interosseous muscle. The ground electrode was positioned between the stimulating electrode and recording electrode. The recording electrode was a bipolar needle electrode. Reflex-evoked muscle potentials (REMP, H-reflex) were recorded using a 1 Hz, 0.5 ms, submaximal stimulus. The stimulating electrode was positioned in the tibial nerve adjacent to the hook, and the recording electrode and ground electrode were positioned in the same tibial nerve site where the M wave was measured. All measurements were performed at least eight times. First, electrophysiological recordings were performed for determining normal values. Electrophysiological recordings were then performed twice more for the experimental group: once before the experiment and once after the test period.
Histopathological analysis
After the experimental period (10 days from the start of the experiment), the dogs were anesthetized with high dose tiletamine/zolazepam (Virbac, France) and given propofol (Choongwae, Korea) to induce euthanasia. They were then perfused transcardially with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in 0.1 M PBS. After perfusion, the lumbar spinal cord (L4), left and right dorsal root ganglia of L4, sciatic nerve, and tibial nerve were quickly removed, post-fixed for 4-6 h in the same fixative at 4℃, and embedded in paraffin. The tissues were sectioned serially to 5 µm thickness with a microtome (Reichert-Jung, Germany), floated onto gelatincoated slides, deparaffinized in xylene, rehydrated in a descending ethanol series, and stained with hematoxylin and eosin. The sections were examined under an Olympus BX51 microscope (Olympus, Japan) attached to an IMT2000 digital camera (iMTechnology, Korea).
Statistical analysis
Paired t-test was done for analysis of body weight and the M wave and H-reflex amplitudes before and after pyridoxine treatment. Statistical significance was set at p < 0.05.
Results
Weight measurement showed that there was weight loss in the treated group. The weight of control group animals was maintained at 5.35 ± 0.21 kg for experimental period. The weight of treated group animals decreased from 4.92 ± 0.40 kg to 4.4 ± 0.43 kg. The difference in body weight before and after pyridoxine treatment was statistically significant (p < 0.05).
All dogs in the treated group developed neurologic abnormalities, characterized especially by ataxia involving first, and most prominently, the hindquarters. Five dogs in the treated group showed proprioceptive abnormalities involving the hindquarters on postural reaction test (wheelbarrowing, hopping, extensor postural thrust, placing, tonic neck reaction, and proprioceptive positioning), starting on the third day of pyridoxine injection. On the fourth day of pyridoxine injection, five affected dogs held their hindlimbs stiffly when standing. These signs remained until the end of the pyridoxine administration period. One dog in the treated group showed proprioceptive abnormalities involving the hindquarters on postural reaction test, starting on the third day of pyridoxine injection. This dog was severely affected. Hindquarter movements were stiff, spastic, and dysmetric, and the dog adopted a broad-based stance when standing. The lack of coordination in this dog became so severe that it occasionally fell, especially when attempting to walk or turn. Except for weight loss and the neurological problems, there were no changes in body conditions among the test animals.
The results of evaluation of the DEMP (M wave) and REMP (H-reflex) in clinically normal dogs are as follows. The mean M wave amplitude (5.4 ± 2.3 mV) was much higher than the mean H-reflex amplitude (0.5 ± 0.3 mV). The mean M wave latency (2.8 ± 0.3 ms) showed that the M wave is an early response, whereas the mean H-reflex latency (16.2 ± 2.5 ms) showed that the H-reflex is a late response.
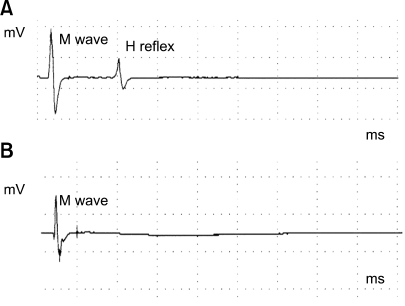
Six dogs in the treated group and two dogs in the control group underwent electrophysiological recordings, through which the M wave and H-reflex were measured. In the experimental group, the amplitude of the M wave was 4.89 ± 0.62 mV before treatment. After treatment, the amplitude of the M wave was 5.04 ± 0.51 mV. M wave amplitude showed no remarkable change after pyridoxine treatment, a finding confirmed in our statistical analysis (p < 0.05). However, there was a remarkable change in the H-reflex after treatment in the experimental group (Fig. 1). Before pyridoxine treatment, the amplitude of the H-reflex was 0.54 ± 0.06 mV. After pyridoxine treatment, there was no consistently detectable H-reflex.
Fig. 1.
On electrophysiological analysis, a control dog showed an H-reflex (A), but a pyridoxine model dog showed no H-reflex (B). Both have normal M waves. The M wave amplitude showed no remarkable change after pyridoxine treatment, a finding which was confirmed in our statistical analysis (p < 0.05). There was a remarkable change in the H-reflex after treatment in the experimental group.
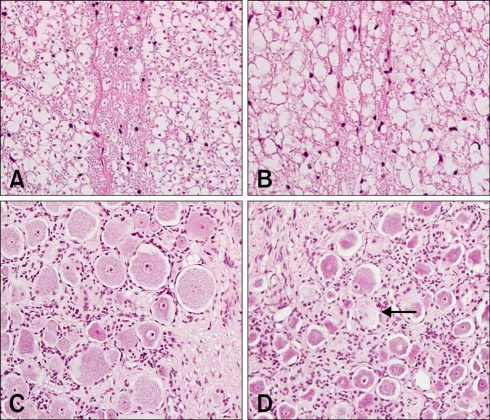
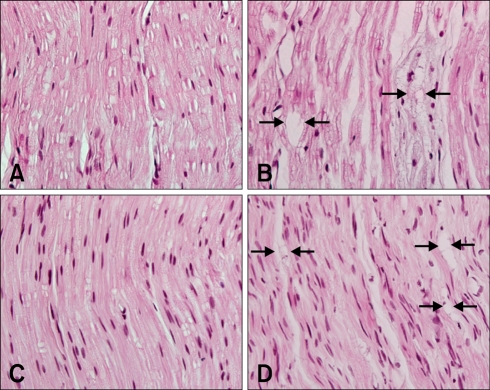
Lesions were observed in the dorsal funiculus of the lumbar spinal cord in experimental animals (L4). The number of axons was reduced, and the myelin was irregular and fragmented, with accompanying vacuolation (Fig. 2B). However, the lateral funiculi, ventral funiculus, and gray matter of L4 were histopathologically normal. Vacuolated myelin was observed in the dorsal root ganglia of L4 (Fig. 2D). Axonal swelling and vacuolation were seen in the sciatic and tibial nerves (Figs. 3B and D).
Fig. 2.
(A) Normal dorsal funiculus in L4. (B) Dorsal funiculus in L4 showing disruption and irregularity of axons and myelin with vacuolation. (C) Normal dorsal root ganglia in L4. (D) Dorsal root ganglia in L4 showing chromatolysis with eccentric location of nucleus (arrow). H&E stain, ×200.
Fig. 3.
(A) Normal sciatic nerve. (B) Sciatic nerve showing axonal swelling with vacuolation (arrows). (C) Normal tibial nerve. (D) Tibial nerve showing axonal swelling with vacuolation (arrows). H&E stain, ×400.
Discussion
Peripheral neuropathies are characterized by motor, sensory, and sympathetic deficits. Sensory neuropathies are frequently associated with diabetes or anticancer therapies [2]. Vitamin deficiency, hypothyroidism, uremia, and characteristically inherited metabolic disorders are also responsible for sensory neuropathies [1]. Therefore, it is very important to develop animal models of sensory neuropathies, in order to conduct preclinical studies of putative neuroprotective and neuroregenerative compounds.
Problematic in the development of experimentally induced sensory neuropathy models are the side effects of the inducing treatment, such as nephrotoxicity for cisplatin, cardiotoxicity for taxol, and simultaneous injury of motor and sensory fibers for acrylamide [7]. Toxicity studies using those drugs have often successfully induced sensory neuropathy [1]. However, the animal model of pyridoxine-induced peripheral neuropathy established in this study was found to be selective for sensory fibers and to be safe for other organs [5,6].
In previous experiments, reduction in body weight was found to be proportionate to reduction in food consumption [5]. In this experiment, we did not measure the amount of food consumption. Therefore, we found no direct correlation in the reduction of food consumption and reduction in body weight.
Neurologic examination is an earlier indicator of neurotoxicity than electrodiagnostic procedures. Therefore, neurologic examinations were performed every day in this study, and EMG recordings were performed before and after treatments.
We chose to use a bipolar needle electrode for EMG recordings, in contrast with electrodes used in other experiments. The bipolar needle electrode is best for detecting correct waves without interfering error waves. However, it is difficult to use in humans because of the pain it causes. We were able to use the bipolar needle electrode in animals because they were anesthetized before the procedure.
The maximal M wave amplitudes were obtained with supramaximal stimulus, and the maximal H-reflex amplitudes were obtained with submaximal stimulus. However, the H-reflex was not completely cancelled out by stimuli supramaximal to the direct response. In humans, the H-reflex is completely cancelled out when stimulation becomes supramaximal. This may be due to the fact that the high spindle density in the interosseous muscles in quadrupeds induces a stronger reflex [12].
In previous rat studies, the H-reflex was measured to demonstrate pyridoxine-induced sensory neuropathy. In dogs, there have been no studies of the H-reflex in pyridoxine-induced sensory neuropathy. However, our study did utilize measurements of the H-reflex.
The EMG recording data obtained during peripheral nerve stimulation in the experimental group were consistent with selective toxicity to sensory nerves.
An earlier study of pyridoxine toxicity in dogs and rats demonstrated lesions with a distribution similar to that seen in the experimental dogs in this study [6,8].
In conclusion, we confirmed that dog models of pyridoxine-induced sensory neuropathy can indeed be constructed. Our method offers a short-term model of sensory neuropathy as an alternative to the already existing long-term model. This was the first trial in which H-reflexes were measured in dogs with pyridoxine-induced neuropathies. Our method is advantageous in that it did not cause systemic toxicity. Further study is needed to confirm pyridoxine-induced toxicity using electron microscopic observation.
Acknowledgments
This work was supported by the Brain Korea 21 program, Korean Research Foundation Grant (KRF-2006-J02902), and the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University.
References
- 1.Callizot N, Warter JM, Poindron P. Pyridoxine-induced neuropathy in rats: a sensory neuropathy that responds to 4-methylcatechol. Neurobiol Dis. 2001;8:626–635. doi: 10.1006/nbdi.2001.0408. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994;35:304–311. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- 3.Dalton K, Dalton MJ. Characteristics of pyridoxine overdose neuropathy syndrome. Acta Neurol Scand. 1987;76:8–11. doi: 10.1111/j.1600-0404.1987.tb03536.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan JP, Gordon MA. Mushroom poisoning. Case reports and a review of therapy. JAMA. 1984;251:1057–1061. doi: 10.1001/jama.251.8.1057. [DOI] [PubMed] [Google Scholar]
- 5.Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. I. Clinical disease. Vet Pathol. 1981;18:745–756. doi: 10.1177/030098588101800605. [DOI] [PubMed] [Google Scholar]
- 6.Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. II. Pathology. Vet Pathol. 1981;18:757–768. doi: 10.1177/030098588101800606. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins AP, Gilliatt RW. Motor and sensory nerve conduction velocity in the baboon: normal values and changes during acrylamide neuropathy. J Neurol Neurosurg Psychiatry. 1971;34:415–426. doi: 10.1136/jnnp.34.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krinke GJ, Fitzgerald RE. The pattern of pyridoxine-induced lesion: difference between the high and the low toxic level. Toxicology. 1988;49:171–178. doi: 10.1016/0300-483x(88)90190-4. [DOI] [PubMed] [Google Scholar]
- 9.Krinke G, Naylor DC, Skorpil V. Pyridoxine megavitaminosis: an analysis of the early changes induced with massive doses of vitamin B6 in rat primary sensory neurons. J Neuropathol Exp Neurol. 1985;44:117–129. [PubMed] [Google Scholar]
- 10.Perry TA, Weerasuriya A, Mouton PR, Holloway HW, Greig NH. Pyridoxine-induced toxicity in rats: a stereological quantification of the sensory neuropathy. Exp Neurol. 2004;190:133–144. doi: 10.1016/j.expneurol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Schaumburg H, Kaplan J, Windebank A, Vick N, Rasmus S, Pleasure D, Brown MJ. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N Engl J Med. 1983;309:445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- 12.Sims MH, Selcer RR. Occurrence and evaluation of a reflex-evoked muscle potential (H- reflex) in the normal dog. Am J Vet Res. 1981;42:975–983. [PubMed] [Google Scholar]
- 13.Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]