Figure 3.
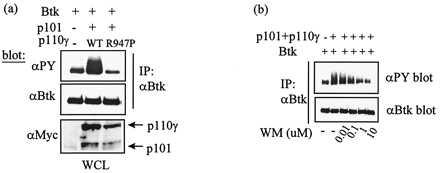
Kinase-inactive p110γ (R947P) and wortmannin treatment block increased Btk tyrosine phosphorylation induced by PI 3-kinase-γ. (a) Rat-2 cells harboring SrcE378G mutant infected with Btk retroviruses, double header retroviruses encoding Btk and p101 in addition to p110γ retroviruses or retroviruses encoding p110γ-R947P mutant were lysed, and Btk was immunoprecipitated with anti-Btk antibody. The immunoprecipitated Btk was run on 8% PAGE. Btk was immunoblotted with monoclonal anti-phosphotyrosine antibody (4G10) or polyclonal anti-Btk antibody, followed by horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies. Whole cell extracts were immunoblotted using an anti-Myc monoclonal antibody (9E10) and visualized with ECL (Amersham). (b) Rat-2 cells harboring the SrcE378G mutant infected with double header viruses encoding Btk and p101 plus retroviruses encoding p110γ were treated with 10 nM, 100 nM, 1 μM, or 10 μM wortmannin 40 h postinfection for 1.5 h before lysis. Tyrosine phosphorylation of immunoprecipitated Btk from wortmannin-treated cells was compared with Btk from untreated Rat-2 cells harboring the SrcE378G mutant and expressing either Btk alone or Btk, p101, and p110γ. The anti-phosphotyrosine blots or anti-Btk blots were produced and detected as described in a.
