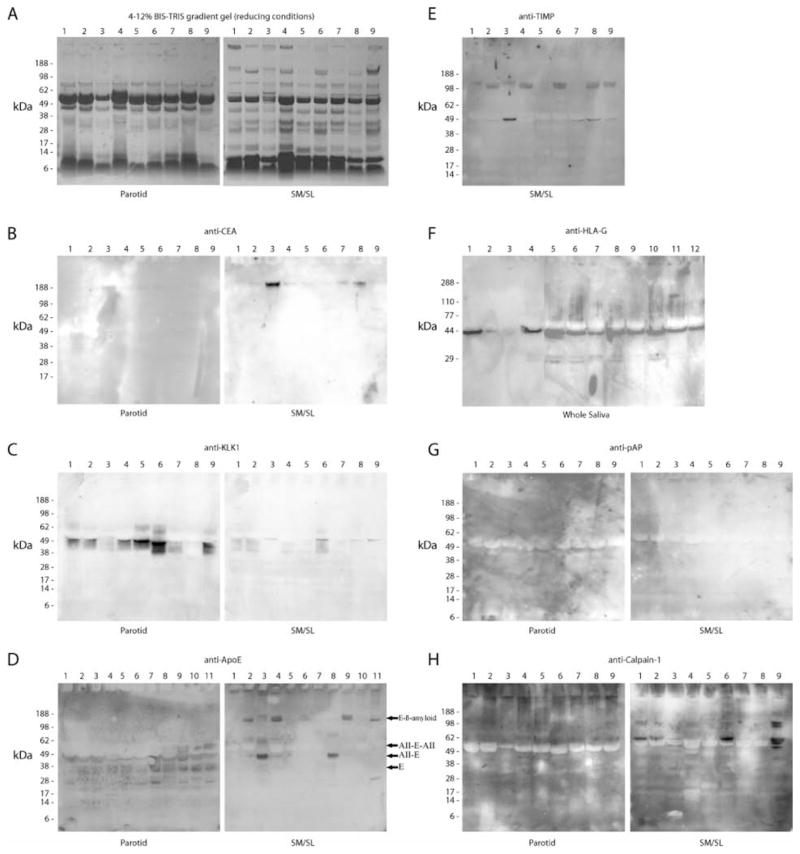
Figure 8.
Detection of novel salivary proteins and those with low sequence coverage. An immunoblot approach was used to confirm the presence in saliva of a portion of the proteins that were identified by using MS approaches. Most of the analyses shown employed parotid and SM/SL saliva samples collected as the ductal secretions from 5 females (lanes 1–5) and 4 males (lanes 6–9). As shown in panel F, whole saliva samples were collected from 10 females (lanes 1, 4–12) and 2 males (lanes 2–3). Proteins were selected for validation based on the number of research groups that reported the identification and the extent of sequence coverage. In general, emphasis was placed on the analysis of novel components and proteins with the least MS evidence of their presence in ductal saliva. (A) Samples were separated on 4–12% BIS-TRIS gradient gels under reducing conditions, and proteins were visualized by staining with Coomassie brilliant blue. Immunoblots detected the following: (B) CEA (IPI00027486, 6% sequence coverage, 1 group reporting), (C) kallikrein-1 (IPI00304808, 89% sequence coverage, 2 groups reporting), (D) apoE (IPI00021842, 21% sequence coverage, 1 group reporting), (E) TIMP-1 (SM/SL only, IPI00032292, 67% sequence coverage, 2 groups reporting), (F) HLA-G alpha chain, a nonclassical class I histocompatibility antigen (whole saliva, IPI00015988; a single peptide was sequenced by 1 research group), (G) prostatic acid phosphatase (IPI00396434, 8% sequence coverage, 1 group reporting), and (H) calpain-1 catalytic subunit (IPI00011285, 15% sequence coverage, 1 group reporting). E, apoE; AII-E, a complex of apoAII and apoE; AII-E-AII, AII-E with an additional apoAII; E-β-amyloid, a complex of apoE and β-amyloid.