Figure 2.
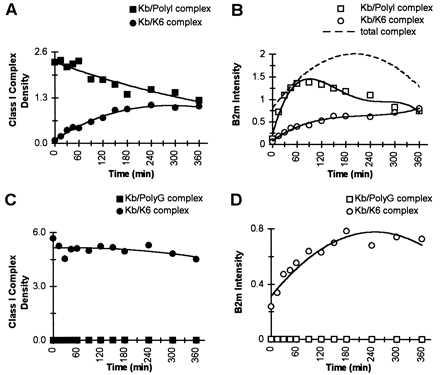
Varying dissociation rates of the class I peptides do not affect the β2m exchange rate. Either purified Kb/PolyI complex (A and B) or Kb/PolyG complex (C and D) were incubated with exogenous 125I-β2m (complex β2m/125I-β2m; 1:1) and with K6 competitor peptide (complex peptide/competitor; 1:>200) in PBS at 37°C. Aliquots were withdrawn at the specified times and analyzed by IEF gel chromatography and visualized by Coomassie staining and by phosphoimage detection of the same gel. (A) The densities of the Coomassie-stained bands for Kb/PolyI complex (▪) and the Kb/K6 complex (•) illustrate K6 peptide exchange over the incubation time; and the t1/2 for the dissociation of PolyI peptide from Kb/PolyI complex is indicated to be 6 hr. (B) The phosphoimage intensities for 125I-β2m, associated either to Kb/PolyI complex (□) or to Kb/K6 complex (○) are compared. The t1/2 for the β2m exchange onto the total amount of Kb/peptide complex is indicated to be ≈30 min (dotted line). (C) The densities of the Coomassie-stained bands for the Kb/PolyG complex (▪) and the Kb/K6 complex (•) illustrate K6 peptide exchange over the incubation time. The t1/2 for the exchange of K6 onto Kb/PolyG complex could not be measured because of the instantaneous dissociation of the prebound PolyG peptide. (D) The phosphoimage intensities for 125I-β2m, associated either to Kb/PolyG complex (□) or to Kb/K6 (○) complex are compared. As the Kb/PolyG complex was instantaneously exchanged into Kb/K6 complex, the data reflect the exchange of labeled β2m onto Kb/K6 complex with a t1/2 of ≈30 min.
