Figure 4.
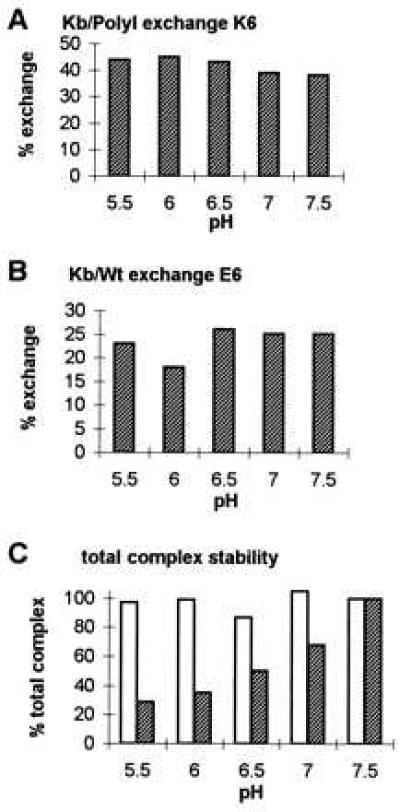
Peptide exchange occurs over a pH range from 5.5 to 7.5 for two different Kb/peptide complexes. Purified Kb/PolyI and Kb/Wt complexes were incubated with K6 (A) or E6 (B) competitor peptide, respectively (complex peptide/competitor; 1:10) in the absence of exogenous β2m in 10 mM Tris adjusted to pH values from 5.5 to 7.5. To assess Kb/PolyI and Kb/Wt complex stability over the tested pH range, the Kb/peptide complexes were incubated without competitor peptide and without β2m for the same incubation time (data not shown). Aliquots were withdrawn at time 0 (data not shown) and 3 hr of incubation at 37°C, analyzed by IEF gel chromatography, and visualized by Coomassie staining. (A) Kb/PolyI complex was exchanged by K6 competitor peptide. The amount of the newly formed Kb/K6 complex is indicated as percentage of exchange (%), as compared with the starting amount of Kb/PolyI complex (100%) at time zero. (B) The exchange of E6 competitor peptide onto Kb/Wt complex is illustrated. The percentage of exchange of newly formed Kb/E6 complex after 3 hr of incubation time is referred to the starting Kb/Wt complex (100%) at time zero. (C) The densities of the Coomassie-stained bands for the total amounts of Kb/peptide complexes, thus Kb/PolyI added to Kb/K6 (empty bars) and Kb/Wt added to Kb/E6 (filled bars), after 3 hr of incubation at 37°C illustrate complex stability over the pH range from 5.5 to 7.5. The maximal values for the two complex combinations at pH 7.5 were set at 100%. For the total Kb/Wt added to Kb/E6 complex, stability was decreased at the lower pH range (5.5 and 6.0). A similar instability was observed for the control Kb/Wt complex incubated in the absence of competitor peptide over the pH range tested (data not shown).
