Abstract
Acute intermittent porphyria (AIP), an autosomal dominant hepatic porphyria due to half-normal hydroxymethylbilane synthase (HMB-synthase) activity, is manifested by life-threatening acute neurological attacks that are precipitated by factors that induce heme biosynthesis. The acute attacks are currently treated with intravenous hemin, but a more continuous therapy is needed, particularly for patients experiencing frequent attacks. Thus, a recombinant AAV8-based serotype vector expressing murine HMB-synthase driven by liver-specific regulatory elements was generated and its effectiveness to prevent the biochemical induction of an acute attack was evaluated in an AIP mouse model. Intraperitoneal administration of the adeno-associated viral (AAV) vector resulted in a rapid and dose-dependent increase of HMB-synthase activity that was restricted to the liver. Stable expression of hepatic HMB-synthase was achieved and wild-type or greater levels were sustained for 36 weeks. When heme synthesis was periodically induced by a series of phenobarbital injections, the treated mice did not accumulate urinary δ-aminolevulinic acid (ALA) or porphobilinogen (PBG), indicating that the expressed enzyme was functional in vivo and prevented induction of the acute attack. Further, rotarod performance and footprint analyses improved significantly. Thus, liver-directed gene therapy provided successful long-term correction of the hepatic metabolic abnormalities and improved neuromotor function in the murine model of human AIP.
Introduction
Acute intermittent porphyria (AIP) is an autosomal dominant inborn error of heme biosynthesis resulting from the half-normal activity of hydroxymethylbilane synthase (HMB-synthase; EC 4.3.1.8).1 Although many AIP heterozygotes are clinically latent, manifesting patients have life-threatening acute neurological attacks that are characterized by severe abdominal pain, hypertension, tachycardia, nausea, motor weakness, and transient psychosis. The acute attacks are precipitated by various drugs, dieting, and hormonal changes, all of which induce mRNA expression and increase activity levels of hepatic 5′-aminolevulinic acid synthase (ALAS1), the first, rate-limiting, and heme-regulated enzyme in the heme biosynthetic pathway. When hepatic ALAS1 is induced, the deficient HMB-synthase activity becomes rate-limiting, resulting in the accumulation of the porphyrin precursors, δ-aminolevulinic acid (ALA) and porphobilinogen (PBG).1,2,3
To date, the pathogenesis of the acute neurological attacks remains unclear, although liver transplantation in several AIP patients completely stopped their recurrent life-threatening attacks.4,5 Although these and other recent studies have suggested that the porphyrin precursors are neurotoxic,6,7 it is notable that the AIP (HMB-synthase deficient) mice develop a chronic peripheral neuropathy in the absence of ALA and PBG accumulation,8 thus supporting the hypothesis that heme deficiency in nervous tissues is also involved in the disease pathogenesis.
Current treatment of the acute attacks involves the intravenous administration of hemin.9 Although patients generally respond well, hemin is rapidly metabolized and its effects are transient. In addition, patients in need of frequent infusions, particularly women who suffer recurrent attacks with their menstrual cycles, are at risk from side effects such as iron overload and phlebitis, which may compromise peripheral venous access. Therefore, an alternative therapeutic approach for AIP that is long-lasting, preventive, and safe, is desirable.
Previously, a mouse model of AIP that has ~30% of wild-type HMB-synthase activity was generated by homologous recombination.10 When the porphyrinogenic drug phenobarbital is administered to these mice, their hepatic ALAS1 activity is induced and ALA and PBG accumulate in their plasma and urine. Systemic administration of recombinant adenoviral vectors containing the HMB-synthase complementary DNA to these mice resulted in increased levels of hepatic HMB-synthase activity, thereby inhibiting the phenobarbital-induced ALA and PBG accumulation.11 However, transgene expression mediated by the first-generation adenoviral vectors rapidly diminished, presumably due to vector shutdown associated with cytotoxic immune responses.12 Thus, more promising vector alternatives are needed, such as the adeno-associated viral (AAV) vectors, which are capable of supporting long-term transgene expression with reduced risk of immunologic consequences.13,14
Here, we evaluated the effectiveness of AAV-mediated gene therapy for AIP using a recombinant AAV8-based serotype vector encoding murine HMB-synthase (designated rAAV2/8-HMBS) under the liver-specific transcriptional control of the human α1-microglobulin enhancer and the human α1-antitrypsin promoter. Intraperitoneal injection of rAAV2/8-HMBS to the AIP mice demonstrated increased levels of hepatic HMB-synthase activity that effectively and continuously prevented the phenobarbital induction of ALA and PBG for over 36 weeks.
Results
rAAV2/8-HMBS-mediated expression is dose-responsive and tissue specific
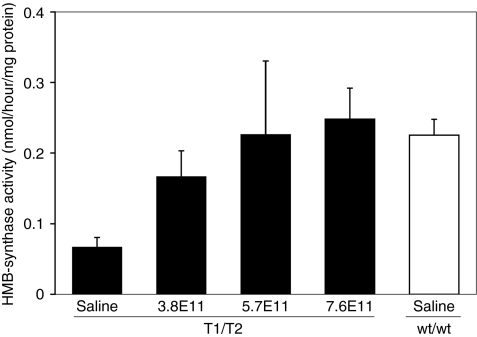
AIP mice were intraperitoneally administered 3.8 × 1011, 5.7 × 1011, or 7.6 × 1011 DNase-resistant particles of rAAV2/8-HMBS and hepatic HMB-synthase levels were determined 1 week later. Although the saline-treated AIP mice had baseline activity of ~30% of wild-type, a dose-dependent increase of hepatic HMB-synthase activity was seen in the rAAV2/8-HMBS-treated mice (Figure 1). The highest vector dose (7.6 × 1011 DNase-resistant particles) achieved HMB-synthase levels slightly greater than mean wild-type levels (Figure 1), thus, this dose was used for all subsequent studies.
Figure 1.
Dose-response of hepatic HMB-synthase expression. AIP mice were administered 3.8 × 1011, 5.7 × 1011, or 7.6 × 1011 drp of rAAV2/8-HMBS and the hepatic HMB-synthase activities were determined 1 week later. Black bars represent HMB-synthase activities detected in the AIP (T1/T2) mice, whereas the white bar represents that of saline-treated wild-type (wt/wt) mice. Data are presented as mean + SD (n = 4). AIP, acute intermittent porphyria.
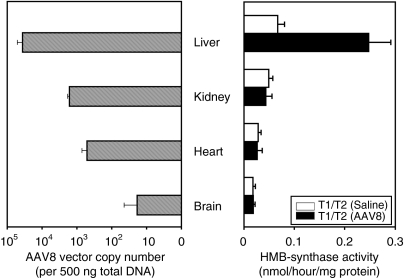
To investigate the tissue specificity of rAAV2/8-HMBS-mediated expression, various tissues including liver, kidney, heart, and brain were isolated 1 week after vector administration and evaluated for AAV vector copy number and HMB-synthase activities. Quantitative PCR analysis detected the highest amounts of rAAV2/8-HMBS vector DNA in the liver, with ~3.6 × 104 copies/500 ng total DNA, followed by the kidney and heart, which had ~1.6 × 103 and ~500 copies/500 ng total DNA, respectively (Figure 2). Not unexpectedly, negligible amounts were detected in the brain. The HMB-synthase activity was increased ~3.5-fold in the liver, whereas the activity in the kidney, which had considerable amounts of the rAAV2/8-HMBS vector, remained at baseline levels (Figure 2). These results indicated that expression of HMB-synthase from the rAAV2/8-HMBS vector, driven by the α1-microglobulin enhancer and α1-antityrpsin promoter, was primarily restricted to the liver.
Figure 2.
Tissue specificity of rAAV2/8-HMBS-mediated HMB-synthase expression. Various tissues from saline- and rAAV2/8-HMBS-treated AIP mice were assayed for HMB-synthase activity (right) and vector copy number (left). The data presented are means + SD (n = 4). Note that vector copy number is shown on a logarithmic scale. AIP, acute intermittent porphyria.
Sustained rAAV2/8-HMBS-mediated hepatic expression of HMB-synthase protects against phenobarbital-induced urinary ALA and PBG accumulation
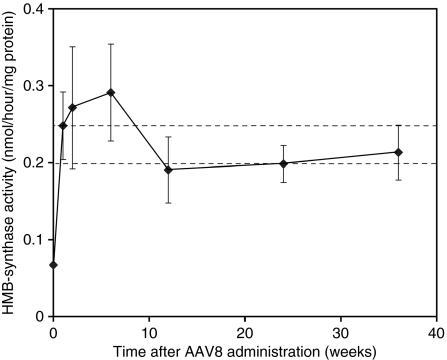
Hepatic HMB-synthase activity was determined in the rAAV2/8-HMBS-treated mice at 1, 2, 6, 12, 24, and 36 weeks after vector administration. Mean hepatic HMB-synthase activity increased to levels slightly greater than wild-type levels by 1 week and continually increased up to 6 weeks, when it achieved maximal mean activity of ~1.3-fold over mean wild-type levels (Figure 3). Thereafter, stable activities within the range of wild-type mice were maintained up to 36 weeks (Figure 3), thus indicating that stable expression of the enzyme was attained using the rAAV2/8-HMBS vector.
Figure 3.
Time course of rAAV2/8-HMBS-mediated hepatic HMB-synthase expression. AIP mice were sacrificed 1, 2, 6, 12, 24, and 36 weeks after vector administration and the hepatic HMB-synthase activities were determined. Results are shown as mean ± SD. Of note, the HMB-synthase activity at week 0 represents baseline activity in the AIP mice. The area between the broken lines represents the range of hepatic HMB-synthase activity detected in wild-type mice (n = 5). AIP, acute intermittent porphyria.
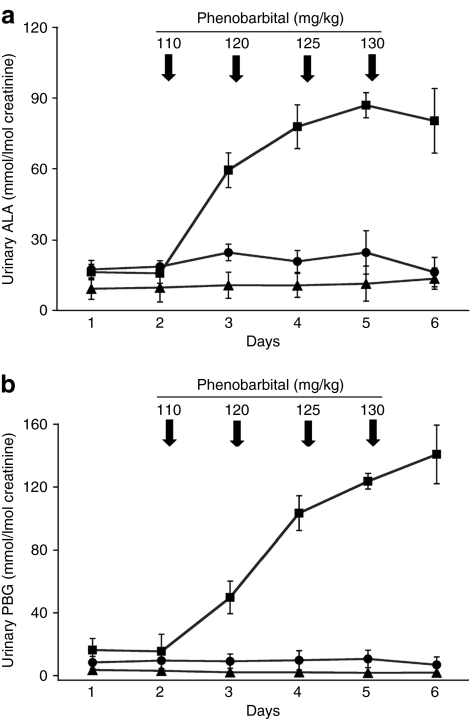
rAAV2/8-HMBS- and saline-treated AIP mice were challenged with intraperitoneal injections of phenobarbital at 2, 10, 22, and 34 weeks after treatment to evaluate whether the expressed HMB-synthase enzyme prevented the biochemical induction of porphyrin precursors. Consistent with previous studies, urinary ALA and PBG increased markedly in the saline-treated AIP control mice, reaching levels of six- and tenfold greater, respectively, than the mean baseline values.10,11 In contrast, phenobarbital induction in the rAAV2/8-HMBS-treated AIP mice did not increase their urinary ALA or PBG concentrations, which remained at levels similar to those in the wild-type mice (Figures 4a,b). This was consistent in all four experiments in which the mice were induced with phenobarbital.
Figure 4.
Urinary porphyrin precursor levels following phenobarbital injections. After collection of two baseline urine samples (days 1 and 2), increasing doses of phenobarbital (110, 120, 125, 130 mg/kg/day) were administered to wild-type (triangles) and saline- (squares) and rAAV2/8-HMBS-treated AIP mice (circles) for four consecutive days, as indicated by the arrows, and the (a) ALA and (b) PBG levels were determined in 24 hour urines. Results from a representative phenobarbital induction performed 22 weeks after vector administration are shown. For each time-point, the means and standard deviations are presented (n = 5). AIP, acute intermittent porphyria; ALA, δ-aminolevulinic acid; PBG, porphobilinogen.
To investigate whether the AAV8-mediated HMB-synthase activity altered the response of hepatic ALAS1 expression to phenobarbital induction, relative ALAS1 transcript levels were determined at baseline and 9 hours after the fourth and final phenobarbital injection (130 mg/kg) at 34 weeks after rAAV2/8-HMBS treatment. Real-time PCR analysis showed that baseline mean ALAS1 expression levels in the saline-treated AIP mice were approximately threefold higher than those of wild-type mice (relative ALAS1 transcript levels: 47.9 and 17.8, respectively), consistent with previous findings.10 Nine hours following the final phenobarbital injection, mean hepatic ALAS1 expression levels in the saline-treated AIP mice were markedly increased, whereas only slight increases were detected in the wild-type mice (230 versus 26.6, respectively). Not only did AAV8 treatment of the AIP mice normalize baseline hepatic ALAS1 levels (47.9 to 11.6), but it also decreased the phenobarbital-induced ALAS1 expression by approximately threefold (230 to 82.0).
rAAV2/8-HMBS therapy improves neuromotor function of AIP mice
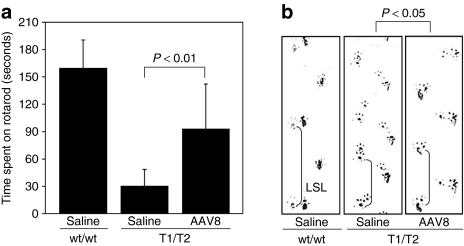
Previously, it was shown that the AIP mice develop chronic progressive neuromotor impairment by 6 months of age.8 Therefore, the impact of rAAV2/8-HMBS treatment on motor coordination and balance skills was assessed by rotarod testing at 24 weeks after vector administration, when the mice were 7 months of age. Although the age- and sex-matched wild-type mice were able to stay on the rotarod for an average of 160 seconds, saline-treated AIP mice averaged 30 seconds (21% of mean wild-type results; Figure 5a), consistent with previous studies.8 The rAAV2/8-HMBS treated mice performed significantly better (P < 0.01), averaging 93 seconds (55% of wild-type; Figure 5a). To evaluate the gait pattern, footprinting was performed at 9 months of age and left stride lengths were measured. Consistent with previous findings, the saline-treated AIP mice had significantly shorter mean left stride length (3.73 ± 0.76 cm; mean ± SD) compared to their wild-type controls (6.63 ± 0.35 cm) (Figure 5b). The rAAV2/8-HMBS-treated AIP mice had a mean left stride length (5.14 ± 0.86 cm) that was shorter than that of wild-type mice but significantly greater than that of the saline-treated AIP mice (P < 0.05; Figure 5b). Thus, rAAV2/8-HMBS therapy improved the neuromotor function of the AIP mice.
Figure 5.
Effect of rAAV2/8-HMBS therapy on neuromotor function. (a) Seven month old male wild-type (wt/wt) and saline- and rAAV2/8-HMBS-treated AIP (T1/T2) mice were evaluated for their performance on a rotarod rotating at 16 rpm, for a maximal time of 180 seconds. The results are expressed as means and standard deviations (n = 5). (b) Gait patterns were analyzed in nine month old mice by footprint analysis. Representative footprints of wild-type and saline- and rAAV2/8-HMBS-treated AIP mice are shown. LSLs were measured and statistically evaluated as described in the “Materials and Methods” section (n = 4–6). AIP, acute intermittent porphyria; LSL, left stride length.
Discussion
AIP is the most common and generally the most severe of the four acute hepatic porphyrias, the others being variegate porphyria, hereditary coproporphyria, and ALA dehydratase deficient porphyria.1 The life-threatening acute neurological attacks in patients with these disorders are precipitated by factors that induce hepatic ALAS1 and/or deplete the heme pool, resulting in the marked accumulation of ALA and PBG.1 Although the pathogenic mechanism underlying the acute neurological attacks remains elusive, the fact that orthotopic liver transplantation cured patients with AIP4,5 and a patient with variegate porphyria15 indicates that restoration of the respective deficient enzymes in the liver alone is therapeutically effective in preventing the acute attacks for the acute hepatic porphyrias.
AIP is an attractive candidate for liver-targeted gene therapy, as a relatively small number of HMB-synthase competent hepatocytes may be sufficient to prevent induction of an acute attack due to the fact that ALA and PBG are small molecules that can diffuse across cell membranes and be metabolized by neighboring transduced cells. As AIP is an autosomal dominant disorder, antibodies against the transgene product will not be raised. Importantly, overexpression of the HMB-synthase enzyme should not be deleterious, as all subsequent enzymes in the pathway are in excess and once hepatic heme concentrations rise, ALAS1 activity will be reduced through the negative feedback mechanism.1
To date, several gene therapy approaches have been investigated for AIP, including nonviral and first-generation adenoviral vectors.11,16 Efforts to use nonviral vectors were unsuccessful, as they were incapable of achieving sufficient HMB-synthase levels due to their poor transfection efficiency in vivo.16 Although the delivery of adenoviral vectors into AIP mice resulted in therapeutic HMB-synthase levels, its expression was transient,11 thus making them impractical for clinical applications. AAV vectors offer a more promising alternative, as they are capable of maintaining high levels of hepatic transgene expression for prolonged periods of time, particularly when regulated by tissue-specific enhancers and promoters.17,18,19 In addition, the recent discovery of novel AAV serotypes, such as AAV8 and 9, has allowed for significantly higher hepatic transduction efficiencies with administration of relatively low viral dosages.20,21 Compared to other viral-based gene therapy vectors, AAV vectors have a favorable biosafety profile, because they are less inflammatory and the wild-type virus is nonpathogenic as well as replication-deficient.13
The effectiveness of AAV8-mediated therapy to correct the metabolic defect of AIP was evaluated in the HMB-synthase deficient mice by administrating a recombinant AAV8-based serotype vector encoding murine HMB-synthase. Transgene expression was driven by the liver-specific α1-microglobulin enhancer and α1-antityrpsin promoter, as this combination previously achieved high levels of hepatic HMB-synthase activity in mice.22 The AAV vectors were delivered to the AIP mice intraperitoneally, because intraperitoneal and traditional tail vein injections achieved comparable levels of hepatic transduction with AAV8 vectors in recent studies.23 Interestingly, intraperitoneal vector administration resulted in a tissue distribution pattern that was similar to that typically observed for intravenous injection of AAV8 vectors24 (Figure 2). Although rAAV2/8-HMBS was delivered to nonhepatic tissues, significantly increased HMB-synthase activity was detected only in the liver (Figure 2), consistent with the use of liver-restrictive regulatory elements. Stable hepatic HMB-synthase expression was attained 1 week after vector administration and activity within the range of wild-type levels was sustained for 36 weeks (Figure 3). Importantly, the rAAV2/8-HMBS-treated mice were continuously protected from the phenobarbital-induced acute attacks, whereas in the saline-treated AIP mice, the urinary ALA and PBG levels—the acute attack “biochemical biomarkers”—were consistently elevated with phenobarbital injections (Figures 4a,b). The fact that hepatic ALAS1 expression levels following phenobarbital induction were considerably lower (~65% less) in the rAAV2/8-HMBS-treated mice than those in the saline-treated AIP mice indicated that the AAV8-mediated HMB-synthase activity effectively reversed the metabolic block in the liver, presumably increasing heme biosynthesis, which in turn downregulated hepatic ALAS1 expression through the negative feedback mechanism. Notably, the phenobarbital-induced hepatic ALAS1 expression levels of the rAAV2/8-treated mice were not decreased to wild-type levels, but were approximately threefold higher, despite the near-normal levels of HMB-synthase activity achieved in the liver. This discrepancy most likely reflects the mosaic cell population of the AAV8-treated liver, which presumably has both transduced (HMB-synthase-sufficient) and untransduced (enzyme-deficient) hepatocytes.
In addition to correcting the hepatic metabolic defect, liver-targeted AAV8 therapy in presymptomatic AIP mice improved neuromotor function, as evidenced by their performance on the rotarod and footprint analysis (Figures 5a,b). Although this is encouraging, it should be noted that there is a distinct difference between the neuropathy that occurs in the AIP mice and human patients. In the mice, the peripheral motor neuropathy develops chronically and progressively in the absence of ALA and PBG accumulation,8 whereas in humans, it typically occurs during an acute attack accompanied by elevated porphyrin precursors, and the symptoms remit once the attack resolves. The reason for this species difference is unknown, but presumably explains why AAV8 therapy only partially improved the neuromotor function in the AIP mice despite the metabolic abnormality in the liver being abolished.
In summary, these studies demonstrate that treatment with the AAV8 liver-targeted vector effectively transduced hepatic cells and provided rapid and prolonged HMB-synthase enzyme activity that continuously protected the AIP mice from the biochemical induction of acute attacks. Further, AAV8 therapy significantly improved neuromotor function in the AIP mice. These studies not only provide the rationale for the development of AAV8-mediated gene therapy for AIP patients with recurrent attacks, but further serve as a treatment model for the other hepatic porphyrias.
Materials and Methods
AAV2/8-HMBS vector construction and production. The full-length murine HMB-synthase complementary DNA of the housekeeping isoform25 was subcloned into the DC-172 expression vector containing the liver-specific α1-microglobulin enhancer and α1-antityrpsin promoter, as previously described.22 The entire expression cassette was excised and cloned into the AAV2 previral plasmid pTR-UF12 (a gift from Michael Linden, Mount Sinai School of Medicine) and designated pTR172-HMBS. Plasmid DNA was purified using the QIAfilter plasmid Giga kit (Qiagen, Valencia, CA) and both inverted terminal repeat sites were confirmed by sequence analysis, following HgaI digestion.
To produce rAAV2/8-HMBS, the pTR172-HMBS plasmid was cotransfected into HEK 293 cells with an adenovirus helper plasmid and a chimeric packaging construct that had the AAV2 rep gene fused to AAV8-derived cap genes. Following purification by column chromatography, rAAV2/8-HMBS was titered for DNase-resistant particles using a real-time TaqMan PCR assay with primers specific to the bovine growth hormone polyadenylation signal sequence.
Animal studies. Animal procedures were reviewed and approved by the Mount Sinai Institutional Animal Care and Use Committee. The T1/T2 AIP mouse model10 was obtained from Urs Meyer, University of Basel, Switzerland, and housed in a barrier facility at the Mount Sinai School of Medicine. These mice are hypomorphic compound heterozygotes; the T1 HMB-synthase allele contains a neomycin gene with a bidirectional phosphoglycerate kinase promoter in exon 1 and the T2 allele has an alternative splice acceptor site in intron 1. Male T1/T2 mice (35–45 days old) were intraperitoneally injected with 0.15 ml of saline solution, with or without rAAV2/8-HMBS. Urines (24 hour) were collected in metabolic cages. Phenobarbital induction was performed as previously described;10 however, the dose was increased to 110, 120, 125, 130 mg/kg/day for four consecutive days. For rotarod analysis, T1/T2 mice were trained for 3 days (two trials per day, 60 seconds maximum per trial) and tested on the forth day, at a rotation speed of 16 rpm (two trials per day, 180 seconds maximum) at 7 months of age. Footprint analysis was performed at 9 months of age, as previously described.10 Analysis of variance was employed for statistical evaluation. Mice were killed at the indicated times by overdose injections of avertin and perfused with phosphate-buffered saline. Tissues from various organs were harvested and snap frozen in liquid nitrogen until use.
HMB-synthase enzyme and porphyrin precursor assays. Tissues were weighed and three volumes/weight of chilled reporter lysis buffer (Promega, Valencia, WI) was added. Homogenization was performed on ice, using a glass homogenizer fitted with a pestle, at a speed of 145 rpm. The samples were centrifuged at 4 °C at 15,000g until the supernatants were clear. HMB-synthase enzyme assays were performed as previously described26 and protein concentrations were measured using the DC protein assay kit, according to the manufacturer's instructions (Bio-Rad, Hercules, CA). One unit of enzymatic activity was defined as that amount of enzyme consuming 1 µmol of PBG per hour. Urinary ALA and PBG levels were determined using the ALA/PBG column kit (Bio-Rad), whereas creatinine was measured using a colorimetric assay based on the picric acid method.27
DNA extraction and quantitation of AAV vector DNA. Total (genomic and plasmid) DNA was extracted from tissues of rAAV2/8-HMBS-treated AIP mice using the Puregene DNA kit (Qiagen). For each sample, 500 ng of total DNA was subjected to TaqMan real-time PCR using primers that specifically annealed to sequences of the murine HMB-synthase complementary DNA (forward primer: 5′-CGCCACCATGTCCGGTAA-3′, reverse primer: 5′-AGCATCGCCACCACAGTGT-3′) and a 6-FAM-TAMRA labeled probe (5′-CGGCCACAACCGCGGAAGAA-3′). PCR conditions used were 50 °C for 2 minutes, 95 °C for 10 minutes, 40 cycles of 95 °C for 15 seconds, 60 °C for 1 minute, and vector copy numbers were quantitated with an ABI Prism 7900 sequence detection system. Absolute AAV vector copy number was determined based on a standard curve made with tenfold serial dilutions ranging from 10 to 107 copies of the AAV vector spiked into 500 ng of wild-type C57/Bl6 tissue genomic DNA. Experiments were performed in triplicates.
RNA extraction and hepatic ALAS1 expression analysis. Total RNA was extracted from livers using TRIzol Reagent (Invitrogen, Carlsbad, CA), treated with DNaseI, and purified by phenol/chloroform extraction. One microgram of total RNA was reverse transcribed with AffinityScript Reverse Transcriptase (Stratagene, La Jolla, CA), using an oligo(dT) primer. Real-time PCR was performed using the SYBR Green method and the following thermocycling conditions: 95 °C for 10 minutes, 40 cycles of 95 °C for 15 seconds, 55 °C for 15 seconds, 72 °C for 30 seconds, 72 °C for 10 minutes. Transcript levels were quantitated with an ABI Prism 7900 sequence detection system. Relative ALAS1 transcript levels were determined (forward primer: 5′-GGATACATTGCCAGCACGAGTTTG-3′, reverse primer: 5′-AGCGTCCATTAGCATCTGCCTCAG-3′) by the comparative Ct method, using murine β-actin (forward primer: 5′-AGGTG ACAGCATTGCTTCTG-3′, reverse primer: 5′-CTGGAGCAGTTTGACG ACAC-3′), α-tubulin (forward primer: 5′-TGCCTTTGTGCACTGGTAT G-3′, reverse primer: 5′-CTGGAGCAGTTTGACGACAC-3′), and ribosomal protein S11 (forward primer: 5′-CGTGACGAAGATGAAGATG C-3′, reverse primer: 5′-GCACATTGAATCGCACAGTC-3′) as internal controls.
Acknowledgments
We thank Michael Linden (Department of Cell and Gene Medicine, Mount Sinai School of Medicine) for kindly providing the previral pTR-UF12 plasmid. This work was supported in part by grants from the National Institutes of Health, including a research grant (5 R01 DK 026824) and a grant (5 MO1 RR00071) for the Mount Sinai General Clinical Research Center from the National Center for Research Resources.
REFERENCES
- Anderson KE, Sassa S, Bishop DF., and , Desnick RJ. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. McGraw-Hill: New York. pp. 2961–3062; 2001. Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. In: Scriver, C, Beaudet, A, Sly, W and Valle, D (eds) [Google Scholar]
- Meyer UA, Strand LJ, Doss M, Rees AC., and , Marver HS. Intermittent acute porphyria–demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972;286:1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Strand LJ, Felsher BF, Redeker AG., and , Marver HS. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci USA. 1970;67:1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonawalla ZF, Orug T, Badminton MN, Elder GH, Rhodes JM, Bramhall SR, et al. Liver transplantation as a cure for acute intermittent porphyria. Lancet. 2004;363:705–706. doi: 10.1016/S0140-6736(04)15646-8. [DOI] [PubMed] [Google Scholar]
- Seth AK, Badminton MN, Mirza D, Russell S., and , Elias E. Liver transplantation for porphyria: who, when, and how. Liver Transpl. 2007;13:1219–1227. doi: 10.1002/lt.21261. [DOI] [PubMed] [Google Scholar]
- Felitsyn N, McLeod C, Shroads AL, Stacpoole PW., and , Notterpek L. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem. 2008;106:2068–2079. doi: 10.1111/j.1471-4159.2008.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis C, Martinez-Bermejo A, Naidich TP, Kaufmann WE, Astrin KH, Bishop DF, et al. Acute intermittent porphyria: studies of the severe homozygous dominant disease provides insights into the neurologic attacks in acute porphyrias. Arch Neurol. 2004;61:1764–1770. doi: 10.1001/archneur.61.11.1764. [DOI] [PubMed] [Google Scholar]
- Lindberg RL, Martini R, Baumgartner M, Erne B, Borg J, Zielasek J, et al. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J Clin Invest. 1999;103:1127–1134. doi: 10.1172/JCI5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen R, Timonen K., and , Mustajoki P. Treatment of the porphyrias. Ann Med. 1994;26:31–38. doi: 10.3109/07853899409147324. [DOI] [PubMed] [Google Scholar]
- Lindberg RL, Porcher C, Grandchamp B, Ledermann B, Bürki K, Brandner S, et al. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat Genet. 1996;12:195–199. doi: 10.1038/ng0296-195. [DOI] [PubMed] [Google Scholar]
- Johansson A, Nowak G, Möller C, Blomberg P., and , Harper P. Adenoviral-mediated expression of porphobilinogen deaminase in liver restores the metabolic defect in a mouse model of acute intermittent porphyria. Mol Ther. 2004;10:337–343. doi: 10.1016/j.ymthe.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Persons DA, Stevenson SC, Frare P, McClelland A, Nienhuis AW, et al. Adenovirus-mediated expresssion of the murine ecotropic receptor facilitates transduction of human hematopoietic cells with an ecotropic retroviral vector. Gene Ther. 1999;6:1456–1468. doi: 10.1038/sj.gt.3300974. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM., and , Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10:309–318. doi: 10.1111/j.1365-2516.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TH., and , Ferry N.2004Liver gene therapy: advances and hurdles Gene Ther 11(Suppl 1): S76–S84. [DOI] [PubMed] [Google Scholar]
- Stojeba N, Meyer C, Jeanpierre C, Perrot F, Hirth C, Pottecher T, et al. Recovery from a variegate porphyria by a liver transplantation. Liver Transpl. 2004;10:935–938. doi: 10.1002/lt.20136. [DOI] [PubMed] [Google Scholar]
- Johansson A, Nowak G, Möller C., and , Harper P. Non-viral delivery of the porphobilinogen deaminase cDNA into a mouse model of acute intermittent porphyria. Mol Genet Metab. 2004;82:20–26. doi: 10.1016/j.ymgme.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Domaradzki ME, Armentano D, Cheng SH, Bishop DF., and , Desnick RJ. Acute intermittent porphyria: vector optimization for gene therapy. J Gene Med. 2007;9:806–811. doi: 10.1002/jgm.1074. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- Beaumont C, Porcher C, Picat C, Nordmann Y., and , Grandchamp B. The mouse porphobilinogen deaminase gene. Structural organization, sequence, and transcriptional analysis. J Biol Chem. 1989;264:14829–14834. [PubMed] [Google Scholar]
- Chen CH, Astrin KH, Lee G, Anderson KE., and , Desnick RJ. Acute intermittent porphyria: identification and expression of exonic mutations in the hydroxymethylbilane synthase gene. An initiation codon missense mutation in the housekeeping transcript causes “variant acute intermittent porphyria” with normal expression of the erythroid-specific enzyme. J Clin Invest. 1994;94:1927–1937. doi: 10.1172/JCI117543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz NW. Textbook of Clinical Chemistry. In: Burtis, C and Ashwood, ER (eds). 3rd edn., W.B. Saunders Company: Philadelphia, PA, pp. 1241–1245 1997.