Table 6.
Asymmetric cyclopropanation of styrene with α-nitro-α-diazocarbonyl compounds 361a–d.
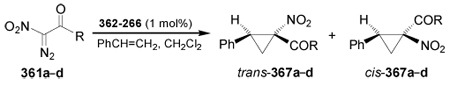 | ||||||
|---|---|---|---|---|---|---|
| Substrate | Catalyst | Aditive | Yield (%) |
Ratio (trans:cis) |
% ee (trans) |
% ee (cis) |
| 361a; R = OMe | 362 | -- | 75 | 86 : 14 | 28 | 13 |
| 361b; R = OEt | 362 | -- | 72 | 83 : 17 | 30 | 0 |
| 361c; R = Ot-Bu | 362 | -- | 68 | 68 : 32 | 41 | 6 |
| 361d; R = Ph | 362 | -- | 64 | 39 : 61 | 31 | 13 |
| 361b; R = OEt | 363 | -- | 71 | 75 : 25 | 13 | 16 |
| 361b; R = OEt | 364 | -- | 76 | 86 : 14 | 33 | 0 |
| 361b; R = OEt | 365a | -- | 89 | 89 : 11 | 2 | 17 |
| 361b; R = OEt | 365b | -- | 74 | 79 : 21 | 8 | 10 |
| 361a; R = OMea | 366a | (BzO)2 | 27 | 90 : 10 | nd | nd |
| 361a; R = OMea | 366a | EDA (20%) | 55 | 90 : 10 | 72 | 51 |
| 361a; R = OMea | 366a | EDA (10%) | 52 | 90 : 10 | 66 | 49 |
| 361a; R = OMeb | 366a | PhNHNH2 | 39 | 90 : 10 | 70 | 49 |
| 361a; R = OMea | 366b | EDA (10%) | 16 | 95 : 05 | 68 | nd |
| 361a; R = OMea | 366c | EDA (10%) | 7 | 95 : 05 | 63 | nd |
in the presence of 5 mol% of Cu(MeCN)4PF6.
Cu(II)OTf2 was used as the copper source
