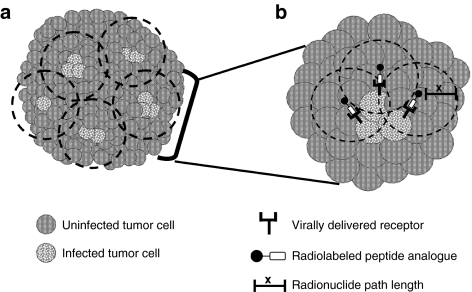
Figure 2.
Model of tumor killing following treatment with combination oncolytic virotherapy and targeted radionuclide therapy. Initial sites of virus infection often occur in distinct foci surrounding blood vessels (if delivered intravenously) or along the needle track (if delivered intratumorally). Preclinical data indicates that spreading of the virus from these initial sites may be limited. Incomplete transduction of large tumors remains a barrier to effective oncolytic virotherapy. (a) Combination oncolytic virotherapy and targeted radionuclide therapy overcomes the barrier of incomplete transduction due to the radiation cross-fire effect. Uninfected cells falling within the area of the path length (depicted by the dashed lines) will be exposed to radiation resulting in DNA damage and subsequent cell death. (b) Enlarged view of the mechanism of killing at each foci of infection. Infected cells express the virally encoded receptor (for example, vvDD-SSTR2) at the cell surface. The receptors are specifically bound by their cognate radiolabeled peptide analogue from which radiation is emitted. Radiation is emitted in all three-dimensions from the site of origin with a maximum tissue penetration distance defined by the path length (x). Virally induced oncolysis and radiation-induced apoptosis will result in significantly increased tumor cell death relative to either therapy alone.