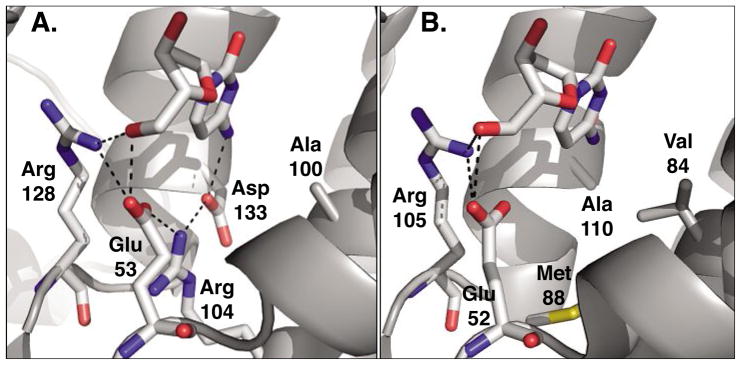
Fig. 4.
Active site comparison of A) human 2′-deoxycytidine kinase (PDB code: 1P6O)[27] and B) 2′-deoxynucleoside kinase from Drosophila melanogaster (PDB code: 1J90).[26] Both enzymes carry 2′-deoxycytidine in the phosphoryl acceptor binding site. Across the type-I kinase family, Arg128 (Arg105) and Glu53 (Glu52) are highly conserved as they form critical hydrogen bonding interactions with the 5′-hydroxyl group of the substrate. In dCK, the hydrogen bonding network is extended to include Arg104 and Asp133 which in turn interacts with the exocyclic amino group on the substrate’s nucleobase. In contrast, the same residues in DmdNK form a large binding pocket which lacks specific contacts and could explain the broader substrate specificity of the fruitfly enzyme. Figures adapted from ref. [28]; created in PyMol (DeLano, Palo Alto, CA).