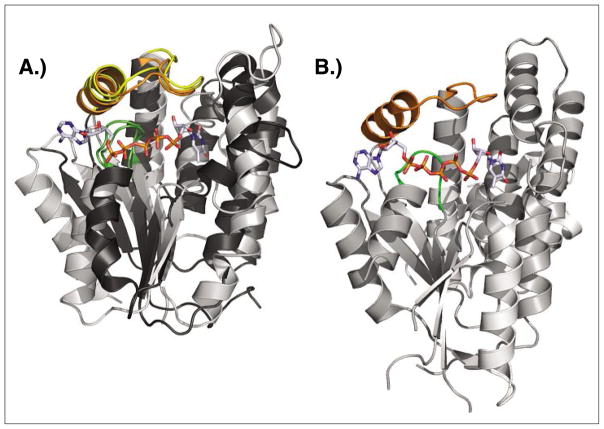
Fig. 8.
Structure comparison of dNK, dNMPK and dual-function kinase. A) Superposition of DmdNK (PDB code: 1OT3)[43] and thymidylate kinase from E. coli (PDB code: 4TMK).[46] Despite overall low sequence identity and strict specificity for either 2′-deoxynucleosides or thymidine monophosphate, the two enzymes have matching functional elements (LID region highlighted in yellow/orange, P-loop shown in green) and striking overall structural similarity. The bisubstrate analog Tp5A (P1-(5′-adenosyl)P5-(5′-thymidyl)pentaphosphate) bound in the active site of the E. coli kinase is shown in sticks B) The thymidine kinase from Herpes Simplex virus (PDB code: 1P7C)[47] is primarly a thymidine kinase, yet can also perform the second phosphorylation step from TMP to TDP. The enzyme carries N and C-terminal extensions but otherwise shares the same core structure with dNKs and dNMPKs including the central β-sheet, the LID region (orange), and the P-loop (green). The binding conformation of the co-crystallized Tp5A analog also closely resembles the orientation seen in the above E. coli structure.