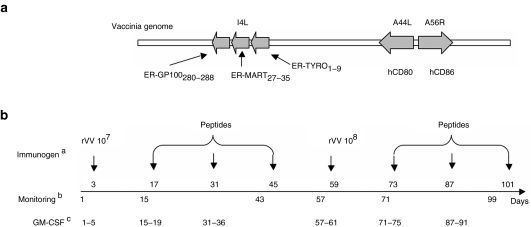
Figure 1.
Recombinant vaccinia virus and design of the study. (a) The recombinant vaccinia virus (rVV) used in this study encodes gp100280–288, Melan-A/MART-127–35, and tyrosinase1–9 tumor-associated antigen epitopes, in the form of fusion peptides targeted to the endoplasmic reticulum (ER), in the nonessential viral locus I4L and human CD80 and CD86 in the nonessential viral loci A44L and A56R, respectively. (b) aImmunogens were administered intranodally at the indicated days of the trial. bBlood samples for monitoring purposes were obtained at the indicated days. cGranulocyte macrophage–colony stimulating factor (GM–CSF) was administered subcutaneously for five consecutive days each week, as indicated.